Abstract
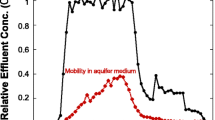
Iron oxide and iron nanoparticles (NPs) have been used effectively for environmental remediation, but are limited in their applications by strong retention in groundwater-saturated porous media. For example, delivery of NPs to large groundwater reservoirs would require large numbers of injection wells. To address this problem, we have explored polymer coatings as a surface engineering strategy to enhance transport of oxide nanoparticles in porous media. We report here on our studies of 2-line ferrihydrite NPs and the influence of poly (acrylic acid) (PAA) polymer coatings on the colloidal stability and transport in natural sand-packed column tests simulating flow in groundwater-saturated porous media. Measurements were also made of zeta potential, hydrodynamic diameter, and polymer adsorption and desorption properties. The coated NPs have a diameter range of 30–500 nm. We found that NP transport was improved by PAA coating and that the transport properties could be tuned by adjusting the polymer concentration. Our results demonstrate that a high stability of oxide particles and improved transport can be achieved in groundwater-saturated porous media by introducing negatively charged polyelectrolytes and optimizing polymer concentrations.








Similar content being viewed by others
References
Chen S (2007) Polymer-coated iron oxide nanoparticles for medical imaging. PhD thesis, Massachusetts Institute of Technology
Chibowski S, Wiśniewska M (2002) Study of electrokinetic properties and structure of adsorbed layers of polyacrylic acid and polyacrylamide at Fe2O3–polymer solution interface. Colloids Surf A 208:131–145
Cornell RM, Schwertmann U (2003) The Iron oxides: structure, properties, reactions, occurrences and uses, 2nd ed. Wiley-VCH, New york
Ghosh S, Jiang W, McClements JD, Xing B (2011) Colloidal stability of magnetic iron oxide nanoparticles: influence of natural organic matter and synthetic polyelectrolytes. Langmir 27:8036–8043
Holsen TM, Taylor ER, Seo YC, Anderson PR (1991) Removal of sparingly soluble organic chemicals from aqueous solutions with surfactant-coated ferrihydrite. Environ Sci Technol 25:1585–1589
Hunter RJ (1981) Zeta potential in colloid science: principles and applications. Academic Press, New York, pp 20–85
Komlos J, Jaffé PR (2004) Effect of iron bioavailability on dissolved hydrogen concentrations during microbial iron reduction. Biodegradation 15:315–325
Komlos J, Peacock A, Kukkadapu RK, Jaffé PR (2008) Long-term dynamics of uranium reduction/reoxidation under low sulfate conditions. Geochim Cosmochim Acta 72(15):3603–3615
M’Pandou A, Siffert B (1987) Polyethyleneglycol adsorption at TiO2–H2O interface: distortion of ionic structure and shear plane position. Coll Surf 24:159–172
Macdonald LH, Moon HS, Jaffé PR (2011) The role of biomass, electron shuttles, and ferrous iron in the kinetics of geobacter sulfurreducens-mediated ferrihydrite reduction. Water Res 45:1049–1062
Orwoll RA, Yong CS (1999) Polymer Data Handbook. Oxford University Press, Newyork, pp 252–253
Tiraferri A, Sethi R (2009) Enhanced transport of zero valent iron nanoparticles in saturated porous media by guar gum. J Nanopart Res 11:635–645
Wu W, He Q, Jiang C (2008) Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res Lett 3:397–415
Acknowledgments
The authors acknowledge support of the Andlinger Innovation Fund of the Andlinger Center for Energy and the Environment at Princeton University. The authors thank Prof. Robert Prud’homme for his valuable discussions and his group for assistance.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiang, A., Yan, W., Koel, B.E. et al. Poly(acrylic acid) coating induced 2-line ferrihydrite nanoparticle transport in saturated porous media. J Nanopart Res 15, 1705 (2013). https://doi.org/10.1007/s11051-013-1705-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1705-3