Abstract
A model of the development and progression of chronic fatigue syndrome (myalgic encephalomyelitis), the aetiology of which is currently unknown, is put forward, starting with a consideration of the post-infection role of damage-associated molecular patterns and the development of chronic inflammatory, oxidative and nitrosative stress in genetically predisposed individuals. The consequences are detailed, including the role of increased intestinal permeability and the translocation of commensal antigens into the circulation, and the development of dysautonomia, neuroinflammation, and neurocognitive and neuroimaging abnormalities. Increasing levels of such stress and the switch to immune and metabolic downregulation are detailed next in relation to the advent of hypernitrosylation, impaired mitochondrial performance, immune suppression, cellular hibernation, endotoxin tolerance and sirtuin 1 activation. The role of chronic stress and the development of endotoxin tolerance via indoleamine 2,3-dioxygenase upregulation and the characteristics of neutrophils, monocytes, macrophages and T cells, including regulatory T cells, in endotoxin tolerance are detailed next. Finally, it is shown how the immune and metabolic abnormalities of chronic fatigue syndrome can be explained by endotoxin tolerance, thus completing the model.
Similar content being viewed by others
Introduction
Research into the cause and treatment of myalgic encephalomyelitis (ME), also known as chronic fatigue syndrome (CFS), has involved the use of 20 different case definitions in common use (Brurberg et al. 2014). The widest definition of CFS is favoured in the UK and only mandates the presence of idiopathic fatigue of variable severity (Sharpe et al. 1991), while the narrowest definition favoured by investigating physicians in the USA mandates the presence of severe incapacitating fatigue, pain, compromised sleep, neurocognitive disability symptoms consistent with autonomic dysfunction and a worsening of global symptoms following even trivial increases in activity (Carruthers et al. 2011). This is an important issue as the use of narrow selection criteria identify patients with far higher levels of physical and cognitive disability than the use of wider criteria (Jason et al. 2012, 2015a, 2016) and criteria variance has been identified as the main factor accounting for the lack of replicated data which has impeded progress in this field (Jason et al. 2015a, b) (reviewed (Morris and Maes 2013a)). Using published criteria, the prevalence of ME/CFS is relatively high, at between 0.2 and 6.4%; together with a low level of employment, reported to be between 27 and 41%, it is clear that this disorder poses a high financial burden on patients and society (Johnston et al. 2013; Rimbaut et al. 2016). It also carries a high cost in terms of symptomology; compared with patients suffering from multiple sclerosis (MS), CFS patients have been reported to suffer from higher levels of symptom severity, higher levels of depression and kinesiophobia, and lower quality of life, lower maximum voluntary muscle contraction and muscle recovery, and lower cognitive performance (Meeus et al. 2016). In spite of this disease burden, research into ME/CFS is relatively low. Indeed, it has been calculated that, in the USA, if the federal research funding for ME/CFS were to take disease burden into account, then, by comparison with the funding pattern for other diseases, the funding for ME/CFS research would need to be increased by at least a factor of 25 (Dimmock et al. 2016).
However, there is a large and accumulating body of evidence reporting the existence of a wide range of biological abnormalities in patients afforded a diagnosis of CFS according to current international consensus criteria (Fukuda et al. 1994), most notably in the neuroendocrine, autonomic, neurological, bioenergetic, redox and immunological domains (Morris and Maes 2013b, c). It is germane to note that, in the USA, the National Institute of Neurological Disorders and Stroke have developed the common data elements for clinical research in mitochondrial disease project ‘to provide clinical researchers with tools to improve data quality and allow for harmonization of data collected in different research studies’ (Karaa et al. 2017, 2018). Common data elements for ME/CFS are being developed along the lines of the following 11 domains: baseline/covariate; fatigue; post-exertional malaise; sleep; pain; neurologic/cognitive/CNS imaging; autonomic; neuroendocrine; immune; quality of life/functional status/activity; and biomarkers. Eleven corresponding subgroups first met in 2017; a related publication will be written in due course.
Early results indicate that increased production of intracellular nuclear-factor 6B and cyclo-oxygenase-2 (COX-2) may be key phenomena in CFS indicating activation of immune-inflammatory pathways in that illness (Maes et al. 2006). In addition, increased production of inducible nitric oxide (NO) synthase (iNOS) coupled with increased IgM responses to NO-adducts such as NO-tryptophan indicate increased nitrosylation of proteins (Maes et al. 2006).
Numerous research teams have reported the presence of an activated but dysregulated immune system with elevated pro-inflammatory cytokines (PICs), T cell anergy, natural killer (NK) cell dysfunction, and Th1, Th2 and, possibly, Th17 lymphocyte biases being repeatedly reported (Brenu et al. 2011; Hornig et al. 2015, 2017; Maes et al. 2012a, b; Milrad et al. 2017; Montoya et al. 2017; Peterson et al. 2015; Russell et al. 2016). There is also evidence of a longitudinal shift in the immune profiles of patients, with an inflammatory phenotype seen in early disease giving way to an anti-inflammatory or immunosuppressed phenotype, indicating activation of the compensatory anti-inflammatory reflex system (Morris and Maes 2013a, b, c) and somewhat reminiscent of the profile seen in endotoxin tolerance in patients who have been ill for many years or even decades (Hornig et al. 2015; Russell et al. 2016). Readers interested in a detailed review of these data are referred to reviews by (Morris and Maes 2013b; Morris et al. 2015a). It should be stressed, however, that there is no evidence of any immune abnormalities in participants afforded a diagnosis of CFS based on any diagnostic schema other than the Fukuda or Canadian criteria (Blundell et al. 2015).
Reported markers of chronic oxidative and nitrosative stress (ONS) include elevated levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS), depleted levels of reduced glutathione (GSH), elevated inducible nitric oxide synthase (iNOS) and oxidatively modified proteins and highly reactive metabolites of lipid peroxidation such as 4-hydroxynonenal and malondialdehyde together with the presence of autoantibodies directed at neoepitopes and the presence of damage associated molecular patterns (DAMPs) (Fulle et al. 2000, 2007; Gerwyn and Maes 2017; Maes 2013; Morris et al. 2015a; Morris and Maes 2013b, c; Rutherford et al. 2016).
Importantly, several research teams have reported that levels of oxidative stress in the muscles of exercising CFS patients are higher than in age- and sex-matched controls and that protective heat shock protein (HSP) responses are impaired (Jammes et al. 2005, 2009, 2011, 2012; Thambirajah et al. 2008). The existence of oxidative stress in individuals afforded a diagnosis of CFS using schemata other than the Canadian or Fukuda criteria is currently uncertain as a literature search failed to uncover any published research investigating this matter.
Chronic ONS is an acknowledged cause of mitochondrial dysfunction (Morris et al. 2017c, d) and hence the fact that impaired synthesis of adenosine triphosphate (ATP), impaired oxidative phosphorylation and damaged or morphologically abnormal mitochondria in striated muscle and peripheral mononuclear blood cells (PMBCs) of patients have all been extensively reported is unsurprising (Morris and Berk 2015; Morris and Maes 2013b; Naviaux et al. 2016; Tomas et al. 2017).
Inflammation, oxidative stress and mitochondrial dysfunction are also recognised drivers of neuroendocrine and autonomic system dysfunction (Kanjwal et al. 2010; Masson et al. 2015; Schultz 2009; Ulleryd et al. 2017), hence the existence of neuroendocrine abnormalities (reviewed (Morris et al. 2017a; Tomas et al. 2013)) and dysautonomia (Lewis et al. 2013; Naschitz et al. 2004, 2006; Newton et al. 2007; Van Cauwenbergh et al. 2014) is to be expected. This is of importance as some 90% of patients diagnosed via the Fukuda criteria have evidence of autonomic dysfunction characterised by increased sympathetic activity, decreased parasympathetic activity and vagal nerve hypoactivity (Beaumont et al. 2012; Lewis et al. 2013; Robinson et al. 2015). The most common manifestation of dysautonomia reported in trial participants is a suppressed and unresponsive heart rate variability (HRV) both during the day and at night (Boneva et al. 2007; Burton et al. 2010; Kadota et al. 2010; Vollmer-Conna et al. 2006). These findings have been confirmed by a large meta-analysis (Martinez-Martinez et al. 2014). Once again it should be stressed that there is no evidence of any abnormal HRV values in patients afforded a diagnosis of CFS via the application of one of a plethora of alternative criteria (Bozzini 2012; Malfliet et al. 2018).
Disrupted patterns of resting state functional connectivity have been repeatedly reported in patients and appear to correlate with levels of fatigue and pain (Boissoneault et al. 2016; Gay et al. 2016; Kim et al. 2015b; Wortinger et al. 2016). The use of voxel-based morphometric analysis of structural magnetic resonance imaging (MRI) brain scans has revealed abnormalities in brain structure and regional volumes (Barnden et al. 2011, 2015; Finkelmeyer et al. 2018; Puri et al. 2012; Shan et al. 2016), including grey matter (GM) and white matter (WM) changes (de Lange et al. 2005, 2008; Finkelmeyer et al. 2018; Okada et al. 2004; Puri et al. 2012; Shan et al. 2016). These findings contrast with earlier work with low resolution MRI and manual analysis which revealed abnormalities in some patients but not others (Perrin et al. 2010) (reviewed (Morris et al. 2017b)).
Systematic studies of cerebral chemistry, using magnetic resonance spectroscopy, have shown evidence of increased regional levels of choline-containing compounds (Chaudhuri et al. 2003; Puri et al. 2002).
The existence of regional or global cerebral hypoperfusion indicative of reduced bioenergetic capacity has also been consistently reported by research teams utilising xenon-computed tomography, arterial spin labelling and high-resolution single-photon emission computed tomography (SPECT) (Biswal et al. 2011; Machale et al. 2000; Patrick Neary et al. 2008; Yoshiuchi et al. 2006). These findings have also been reported in large studies utilising older SPECT techniques either globally (Ichise et al. 1992; Schwartz et al. 1994) or regionally (Costa et al. 1995; Goldstein et al. 1995) but the results in studies with far fewer participants have been negative (Fischler et al. 1996; Peterson et al. 1994).
Unsurprisingly, given the evidence regarding neuroimaging abnormalities above, a meta-analysis of 50 studies has confirmed the existence of widespread cognitive dysfunction in patients, which are at their worst in the domains of attention, memory, reaction times, information processing and reasoning (Cockshell and Mathias 2010). These findings are also discussed in two excellent narrative reviews by (Cvejic et al. 2016; Shanks et al. 2013) and global decreases in cognitive and executive functions have recently been reported in adults and adolescents irrespective of sex (Nijhof et al. 2016; Santamarina-Perez et al. 2014). It is interesting that several research teams using functional MRI (fMRI) have noted that cognitive dysfunction worsens in patients with increased effort and/or exercise, as such findings may be of relevance from the perspective of pathogenesis and/or pathophysiology (Cook et al. 2017; DeLuca et al. 2004; Lange et al. 2005; Tanaka et al. 2006). It is also noteworthy that a recent large study primarily containing participants with CFS confirmed the results of earlier research indicating that there was scant evidence of global cognitive dysfunction in patients diagnosed via alternative criteria (Hughes et al. 2018).
Many patients complain of ‘brain fog’, which is often described as slow thinking, difficulty focusing, slow thinking, lack of concentration, confusion, forgetfulness, or, sometimes, hazy thought processes (Ocon 2013). From a more objective perspective, subjective brain fog can be described as a constellation of symptoms that include impaired cognition, loss of long- and short-term memory, and a reduced ability to concentrate and engage in multiple low-level tasks at once (Theoharides et al. 2015). It is important to note that this phenomenon is not confined to CFS patients but also characterises patients with autism spectrum disorders, coeliac disease, postural orthostatic tachycardia syndrome, as well as patients with mild cognitive impairment and a range of neuroprogressive illnesses (reviewed (Theoharides et al. 2015)). The causes of brain fog are not fully delineated but there is accumulating evidence that this symptom complex may relate to autonomic dysfunction and/or decreased regional cerebral blood flow (rCBF) in the context of ongoing neuroinflammation (Ocon 2013; Ross et al. 2013; Theoharides et al. 2015).
Several research teams have reported changes in the DNA sequences or expression of numerous genes involved in the delivery of the immune response and the regulation of metabolic and bioenergetic pathways in patients diagnosed according to the Fukuda criteria (Morris et al. 2016b). Examples of dysfunctional or abnormally expressed genes compared with matched controls include NFKB1, IL6, IL1A, TNF, IL17A, IL7, CXCL8 (formerly IL8), INFG, IRF3, TLR4, CD14, STAT5A, HSPA2, P2RX7, ATP5J2, GZMA, COX5B, DBI, PSMA3, PSMA4, HINT, ARHC, HLA-DQB1-AS1, RIPK3 and DEFA1 (Carlo-Stella et al. 2006; Gow et al. 2009; Kerr et al. 2008a, b; Light et al. 2009, 2012, 2013; Nguyen et al. 2017; Saiki et al. 2008; Shimosako and Kerr 2014; White et al. 2012; Zhang et al. 2010). There is also evidence of abnormalities in the mitochondrial genome of patients as a research team has recently reported the presence of polymorphisms in the mitochondrial DNA (mtDNA) of their trial participants which were associated with increased symptom severity (Billing-Ross et al. 2016). These findings were reiterated in (Hanson et al. 2016). These are interesting observations as this study contained 196 patients who were recruited via criteria which mandated the existence of what many researchers view as the defining characteristic of CFS, namely an exacerbation of symptoms following even trivial increases in activity (Morris and Maes 2013a).
The observation that mtDNA polymorphisms appear to influence the severity of CFS is consistent with observations in other disease areas where such polymorphisms increase the susceptibility to the development of metabolic and neurodegenerative diseases and susceptibility to microbial infection (review (Hendrickson et al. 2008)). Polymorphisms in mtDNA also play a role in structuring the composition of the microbiota and determining the levels of IgG and IgM autoantibody production (Ma et al. 2014; Zhou et al. 2017). This may be of pathophysiological relevance in the light of data demonstrating elevated IgA and IgM responses to lipopolysaccharide (LPS)/antigens of Gram-negative gut commensal bacteria and gut dysbiosis in patients afforded a diagnosis of CFS via the Fukuda criteria (Maes et al. 2006; Morris et al. 2016b; Morris and Maes 2013b). Mutations in mtDNA can increase levels of inflammation and oxidative stress (I&OS) via direct effects on the innate immune system involving PIC production and NF-κB activity and hence can influence the intensity of the immune response (Imanishi et al. 2013; Ishikawa et al. 2010; Novak and Mollen 2015).
There is also evidence of abnormalities in the epigenetic regulation of gene expression in CFS patients diagnosed via narrow criteria, most notably in gene promoter methylation patterns and elevation of microRNAs (miRNAs) involved in the regulation of the immune system (Brenu et al. 2014a; de Vega et al. 2014, 2017; Petty et al. 2016; Vangeel et al. 2015, 2018). The work of de Vega and others is of particular interest as these authors also selected patients according to criteria mandating the presence of post-exertional malaise and examined global patterns of gene methylation rather than a single gene as was the case for Vangeel and colleagues (de Vega et al. 2014, 2017; Vangeel et al. 2015, 2018).
Importantly, de Vega and others reported a global hypomethylation of cytosine residues in the promoter regions of immune system-related genes consistent with a chronically activated but dysregulated immune system, and abnormal patterns of DNA methylation in genes regulating metabolic pathways and various aspects of cellular homeostasis (de Vega et al. 2014, 2017). The work of (Vangeel et al. 2015, 2018) is also of interest as the pattern of hypomethylation of the glucocorticoid receptor gene NR3C1 1F region suggests an activated hypothalamic-pituitary-adrenal (HPA) axis in an attempt to counter peripheral inflammation rather than a blunted HPA response reported in people diagnosed with CFS according to wider criteria (reviewed (Morris et al. 2017a)). The lack of association between childhood trauma and levels of methylation reported by these authors in studies where participants universally reported this phenomenon is also of interest as these patients were diagnosed according to the Fukuda criteria whereas studies which have reported a significant but slight association between childhood trauma and CFS involved participants recruited via alternative criteria (reviewed (Morris et al. 2017a)).
Abnormalities in miRNA levels in CFS patients diagnosed according to the Fukuda criteria may also suggest dysregulation of immune and metabolic pathways. For example, upregulated hsa-miR-127-3p, hsa-miR-142-5p and hsa-miR-143-3p was reported by (Brenu et al. 2014a) in an analysis of whole blood profiles and elevated expression of hsa-miR-99b, hsa-miR-330, hsa-miR-126 and hsa-miR-30c was reported by (Petty et al. 2016) in an analysis involving NK cells and monocytes. miR-99b upregulation is involved in immune downregulation in macrophages and DCs by reducing levels of IL-6, IL-12 and IL-1β upregulated in response to an infection (Singh et al. 2013; Zheng et al. 2015). miR-127-5p upregulation exerts an immunosuppressive effect by inhibiting the phosphorylation and subsequent translocation of p65 into the nucleus leading to the inhibition of NF-κB signalling and the downregulation of c-Jun N-terminal kinase (JNK)/p38 and reduced levels of PICs (Huan et al. 2016; Park et al. 2013). miR-30 also acts to suppress the immune response by inhibiting the toll-like receptor (TLR)/myeloid differentiation primary response 88 (MyD88) signalling pathway (Wu et al. 2017). Upregulation of miR-143 and miR-142-5 also signals a downregulated immune response as the production both molecules is increased following transforming growth factor beta 1 (TGF-β1) activation and plays positive and inhibitory roles in signalling pathways instigated by this cytokine (Cheng et al. 2014b; Long and Miano 2011; Ma et al. 2016; Yu et al. 2017). miR-126 is an effector of TGF-β1 signalling and regulates the activity of the PI3K/Akt signalling pathway (Guo et al. 2008, 2016). This molecule also regulates multiple aspects of the immune response in macrophages and monocytes, plays a major role in the preconditioning of the immune system against future infections with the same pathogen and activates mechanistic (or mammalian) target of rapamycin kinase (mTOR) and glycogen synthase kinase 3 (GSK-3) (Ye et al. 2013) (reviewed (Ferretti and La Cava 2014; Bai et al. 2014)). In addition, miR-126 regulates cytosolic TLR signalling and modulates the duration and intensity of such signalling in DCs (Rogers and Herzog 2014). Finally, miR-330 also regulates the activity of the PI3K/Akt signalling pathway and also exerts an inhibitory effect on T cell proliferation and NK cell activation (Grigoryev et al. 2011; Kim et al. 2015a; Petty et al. 2016).
Several pathogens have been associated with the development of CFS and evidence of chronic virus infections have been repeatedly reported in the gastrointestinal (GI) tract (Chia et al. 2010; Chia and Chia 2008), serum (Clements et al. 1995) and muscle (Cunningham et al. 1991; Gow and Behan 1991; Lane et al. 2003). Pertinently, these latter findings were not replicated in patients diagnosed according to alternative criteria (Swanink et al. 1994). Several authors have reported the presence of activated Epstein-Barr virus, human herpesvirus 6, cytomegalovirus and parvovirus B19, but these findings are not universal even in patients diagnosed according to narrow criteria (Morris and Maes 2013b) reviewed (Morris et al. 2016d)). Hence it is difficult to conclude that persistent or chronic infections are at the root of CFS/ME/SEID, but of course they could be in some patients. However, many patients have a history of a severe infection before the development of their symptoms (Gow et al. 2009; Hickie et al. 2006; Stormorken et al. 2015; White 2007; Zhang et al. 2010).
In this context it is noteworthy that the intensity of the immune response is not determined solely by the virulence or otherwise of an invading pathogen but by genetic and epigenetic variation in immune response genes (Bronkhorst et al. 2013; Morandini et al. 2016; Rautanen et al. 2015; Smelaya et al. 2016). Epigenetic variation in immune response genes also plays a major role in determining the development of DAMPs in an individual post-infection (reviewed (Morandini et al. 2016)). This may be pertinent from the perspective of the aetiology of CFS, as the production or presence of these molecules can at least in some circumstances ‘convert’ an acute pathogenic infection into a state of escalating chronic systemic inflammation, and hence this mechanism could conceivably underpin the development of chronic symptoms in CFS patients diagnosed according to the Fukuda criteria (Lucas and Maes 2013; Lucas et al. 2015). However, could this relatively simple concept explain all the observations relating to CFS reviewed above? Accordingly, this paper aims to answer this question in the context of developing an explanatory model of illness development and progression, commencing with a proposed mechanism explaining the development of chronic systemic inflammation, oxidative and nitrosative stress (I&ONS) following a pathogen invasion in genetically predisposed individuals.
Acute infection and the development of chronic I&ONS: The role of DAMPs
The relationship between polymorphisms in immunity-related genes and the intensity of the immune response, irrespective of pathogen virulence, has been repeatedly demonstrated (Cvejic et al. 2014; Helbig et al. 2005; Piraino et al. 2012; Vollmer-Conna et al. 2008). Equally, the relationship between an exaggerated immune response and increased production of DAMPs stemming from cellular stress and tissue damage is well documented (Fichna et al. 2016; Kakihana et al. 2016; Rittirsch et al. 2008; Wiersinga et al. 2014).
Mechanistically, the genesis of DAMPs following TLR or nucleotide-binding oligomerisation (NOD)-like receptor activation by a range of pathogens in the context of a hyper-responsive immune system involves prolonged or excessive activation of NFκB and other transcription factors such as nuclear factor of activated T cells and activated protein-1. This leads to upregulation of macrophage and monocyte PICs and activation of T and B lymphocytes (Lucas et al. 2015; Morris et al. 2015a). Abnormally elevated PIC production in turn leads to elevated upregulation of iNOS and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, leading to the production of superoxide, nitric oxide and peroxynitrite, leading to further upregulation of NF-κB and hence further increases in PICs, ROS and RNS levels (Morris et al. 2015a; Morris and Maes 2014). This bidirectional self-amplifying association between the development of chronic systemic inflammation and chronic ONS is sometimes described as an ‘autotoxic loop’ (Ortiz et al. 2013; Reuter et al. 2010). Excessive levels of ROS and RNS can lead to damage to proteins, lipids and DNA and the formation of oxidative specific epitopes and products of lipid peroxidation, which function as DAMPs capable of activating TLRs on cell membranes and cytosolic pathogen recognition receptors (PRRs) (Bowie 2013; Leibundgut et al. 2013; Miller et al. 2011; Uchida 2013).
Importantly, several research teams have reported the presence of such DAMPs in CFS patients (Brkic et al. 2010; Maes et al. 2011; Maes and Leunis 2014; Richards et al. 2007; Tomic et al. 2012; Wang et al. 2014). In addition, there is accumulating evidence indicating that an environment of chronic ONS leads to release of mitochondrial components into the cytosol which also have the capacity to activate cytosolic PRRs and logically are categorised as mitochondrial DAMPs (reviewed (Nakahira et al. 2015)). There are a range of molecular entities which fall into this category other than mtDNA and one such species is cardiolipin (Wenceslau et al. 2014). This is of importance from the perspective of this paper as immunogenic cardiolipin has been repeatedly detected in CFS patients diagnosed according to internationally agreed criteria (Hokama et al. 2008, 2009). The work of Hokama and others would appear to be especially noteworthy as the study contained 320 participants satisfying the requirements of the Fukuda criteria (Hokama et al. 2008).
Research investigating the role of DAMPs in human diseases is advancing apace and the categorisation of these molecules (Jammes et al. 2009) as uniquely proinflammatory entities is changing; one such change which may be relevant to the pathogenesis of CFS is the realisation that HSPs, once thought to be exclusively proinflammatory, have a key anti-inflammatory function and play a crucial role in restraining the intensity and/or duration of the immune response (van Eden et al. 2012) (reviewed (van Eden et al. 2017)). This is pertinent given that HSP production appears to be deficient in CFS patients, and the HSPs produced appear to be dysfunctional, which could potentially provide another mechanism underpinning a prolonged and/or exaggerated immune response to pathogen invasion and other sources of inflammation such as stress, medical comorbidity and lifestyle factors in such patients (Elfaitouri et al. 2013; Jammes et al. 2005, 2009, 2011, 2012).
Chronic engagement of TLRs by DAMPs leads to the development of a positive feedback loop, whereby increasing tissue damage caused by elevated PICs, ROS and RNS perpetuates and escalates pro-inflammatory responses, leading to a state of chronic inflammation, ONS, mitochondrial dysfunction and glial cell activation (Drexler and Foxwell 2010; Goh and Midwood 2012; Morris and Berk 2015; Piccinini and Midwood 2010). Unsurprisingly, chronic engagement of TLRs, NOD-like receptors and retinoid acid-inducible gene I (RIG-I)-like receptors is implicated in the pathogenesis and pathophysiology of systemic lupus erythematosus (SLE), rheumatoid arthritis and MS (review (Drexler and Foxwell 2010; Goh and Midwood 2012; Piccinini and Midwood 2010)). Pertinently, the presence of DAMPs can also lead to chronic activation of the inflammasome (Anders and Schaefer 2014), which is also implicated in the development of neuro-inflammation and abnormal central nervous system (CNS) signalling characteristic of neurodegenerative and neurodevelopmental disorders (Singhal et al. 2014; Tan et al. 2013).
Consequences of chronic systemic I&ONS
Increased intestinal permeability and translocation of commensal antigens into the circulation
Chronically elevated I&ONS in the guise of increased levels of ROS, RNS and PICs induces marked increases in intestinal epithelial barrier permeability (Al-Sadi et al. 2009; Banan et al. 2003; Lee 2015; Tian et al. 2017), ultimately leading to translocation of Gram-negative bacterial LPS and a range of other commensal antigens such as peptidoglycan and flagellin, from the gut lumen into the intestinal mucosa (Lucas et al. 2015; Morris et al. 2016b). Such events lead to the creation of a self-amplifying pattern of localised and then systemic inflammation via several different routes (Delzenne and Cani 2011; Zhang and Zhang 2013).
In the first instance, the presence of LPS in the colon exacerbates inflammation in the intestine and depletes levels of regulatory T cells (Tregs), resulting in increased PIC levels (Im et al. 2012). In addition, increased an concentration of colonic LPS provokes further increases in epithelial tight junction permeability by stimulating the increased synthesis and release of the chemokine IL-8 by intestinal epithelial cells and upregulation of TLR4 on the surface of enterocytes (Angrisano et al. 2010; Guo et al. 2013). Translocated LPS also increases tight junction permeability by inducing increased enterocytic expression of TLR4 and CD14 and changes in the location of their respective proteins (Guo et al. 2013).
The development of gut inflammation interacts with dysbiosis of the microbiome and provokes the recruitment of proinflammatory macrophages into mucosal tissue which exacerbates localised inflammation, further increasing intestinal permeability to the point that enables the translocation of LPS, peptidoglycan and flagellin into the bloodstream (Delzenne and Cani 2011; Zhang and Zhang 2013). This latter phenomenon can have serious consequences in terms of initiating, or in this instance exacerbating, systemic inflammation via the activation of TLR4 and TLR2 on antigen-presenting cells and increasing levels of PICs, ROS and RNS (Morris et al. 2015a, b). The pathogenic consequences of LPS translocation are highlighted by replicated data demonstrating that this phenomenon is the cause of chronic systemic immune activation and I&OS seen in HIV seropositive patients in the absence of viraemia and otherwise well controlled on highly active antiretroviral therapy (Brenchley and Douek 2008; Shan and Siliciano 2014). LPS translocation into the systemic circulation is also held to be the cause of the metabolic endotoxaemia increasingly considered to be a major element in the pathogenesis of a wide range of inflammatory conditions and illnesses such as metabolic syndrome, type 2 diabetes mellitus and MS (Cani et al. 2008, 2009; Puddu et al. 2014; Riccio and Rossano 2015). From the perspective of a model of the pathogenesis and pathophysiology of ME/CFS, the important point is that the advent of LPS translocation in an environment of pre-existing I&ONS would be expected to increase the levels of all these parameters.
Development of autonomic dysfunction
A plethora of human studies have established a causative association between indices of increased systemic inflammation, most notably elevated C-reactive protein and IL-6, and low and unresponsive HRV in a range of inflammatory and infectious illnesses such as MS, type 2 diabetes mellitus and sepsis (de Castilho et al. 2017; Stuckey and Petrella 2013; Studer et al. 2017; Tateishi et al. 2007). More specifically, translocated LPS-mediated systemic inflammation is an acknowledged cause of low and unresponsive HRV in people with metabolic endotoxaemia, which is of relevance given the existence of this phenomenon in many CFS patients (Jan et al. 2010; Lehrer et al. 2010; Morris et al. 2016b). However, the suppressive effect of LPS on HRV is not limited to its effects on systemic inflammation as there is evidence that LPS may also invoke systemic autonomic dysfunction via direct effects on TLR4 receptors on microglia in the paraventricular nucleus of the hypothalamus (Masson et al. 2015; Okun et al. 2014) and the brainstem (Ogawa et al. 2011). The effects of LPS in this instance appear to be mediated via cross-talk with angiotensin II and the ultimate effect is the activation of microglia with resultant increases in I&OS throughout the hypothalamus (Biancardi et al. 2016; Ogawa et al. 2011; Wang et al. 2009).
The relationship between systemic inflammation and HRV is important in a wider context as it is a measure of suppressed vagal nerve activity and a dysfunctional cholinergic anti-inflammatory reflex (reviewed (Huston and Tracey 2011)) and hence is accepted as a surrogate marker of global autonomic dysfunction as the vagus nerve acts on both the cardiac sinoatrial node and the reticulo-endothelial system (Borovikova et al. 2000) (reviewed (Herlitz et al. 2015)). It is also of interest that given the existence of data demonstrating that the extent of HRV suppression correlates with the levels of systemic inflammation (Durosier et al. 2015; Herry et al. 2016) (review (Cooper et al. 2015)), that HRV parameters could act as surrogate markers of systemic inflammation, which could potentially prove to be objective biomarkers for CFS patients in the early stages of their illness (Durosier et al. 2015). However, low HRV values are also seen in patients suffering from major depressive disorder (Brunoni et al. 2013), possibly as a result of associated high levels of peripheral PICs (reviewed (Berk et al. 2013)) and hence HRV values are unlikely to be able to differentiate patients with CFS from those with a similar symptom presentation as a result of major depressive disorder.
Development of neuroinflammation, neurocognitive and neuroimaging abnormalities
Development of neuroinflammation
There is accumulating data demonstrating a causative association between the development of chronic systemic inflammation and disruptions in resting state connectivity, the development of GM and WM atrophy and lesions, and reduced cerebral perfusion (Adam et al. 2013; Felger et al. 2015; Labrenz et al. 2016; Lekander et al. 2016; Marsland et al. 2015; Riverol et al. 2012; Sankowski et al. 2015; Sonneville et al. 2013). There is also a large and increasing body of evidence indicating a causative relationship between the presence of chronic systemic inflammation and the existence of cognitive disability in patients diagnosed with a range of neuroinflammatory, neurodegenerative and neuroprogressive conditions (Gorelick 2010; Marsland et al. 2015; Sartori et al. 2012).
Inflammatory signals can reach the brain via humoral and neural routes to activate the HPA axis (Morris and Berk 2015). The humoral route involves direct or indirect cytokine signalling, either through direct access to the brain via regions where the integrity of the blood-brain barrier (BBB) is compromised or absent, such as the choroid plexus or other circumventricular organs (CVOs) (Morris et al. 2013), or by direct entry via saturable BBB transport systems or an indirect induction of cytokines and other inflammatory mediators, such as prostaglandins, and their subsequent release into the CNS parenchyma or via provocation of an increase in BBB permeability (Morris et al. 2015b; Seruga et al. 2008). The neural route involves direct stimulatory action of PICs on peripheral afferent neurons of the vagus nerve (Goehler et al. 2000; Johnston and Webster 2009).
Entry of PICs into the brain can have profound pathological consequences either directly or indirectly. There is now overwhelming evidence that transduced inflammatory signals provoke the development of chronic neuroinflammation secondary to the sequential activation of microglia and astrocytes. Activated microglia secrete a range of neurotoxic molecules such as tumour necrosis factor (TNF)-α, IL-6, IL-1β, ROS, RNS, COX-2, prostaglandin E2 (PGE2), glutamate and, in some cases, quinolinic acid (Morris et al. 2015b; Morris and Maes 2013b). Moreover, the release of PICs can in themselves act as independent sources of RNS, primarily NO, and other neurotoxins via their capacity to upregulate iNOS, COX-2 and PGE2 (Morris et al. 2015a; Sofroniew and Vinters 2010). Unsurprisingly, the production of these neurotoxins can exert profound and detrimental effects on neurotransmitter systems, and neural integrity and function (Morris et al. 2015b).
Activated microglia and subsequent release of PICs and glutamate, combined with reduced glutamate reuptake by activated astrocytes, can lead to the development of glutamate neurotoxicity with resulting damage to glutamatergic neurones and disruption to glutamatergic neurotransmission (Noda 2016; Robel et al. 2015; Takeuchi et al. 2006), while the I&OS generated by such activation damage A9 dopamine and A6 noradrenaline neurones thereby respectively disrupting dopaminergic and noradrenergic neurotransmission (Nagatsu and Sawada 2006; Tripathy et al. 2015). There is also an accumulating body of evidence indicating that elevated ROS and RNS in the CNS can inhibit dopaminergic neurotransmission by inhibiting dopamine receptors (Morris et al. 2017d). Elevated CNS PIC levels, and subsequent activation of the p38 mitogen-activated protein kinase (MAPK) signalling system, can also adversely affect the synthesis, reuptake and release of serotonin (Miller et al. 2013). Elevated PICs in the CNS also provoke the activation of the tryptophan catabolite pathway, depleting levels of tryptophan, which is the precursor of 5-HT, as well as creating another dimension of neuropathology via the synthesis of several neurotoxic tryptophan catabolites including the potentially neurotoxic quinolinic acid (Miller et al. 2013) (reviewed (Morris et al. 2016e)).
There are also some data to suggest that activated microglia inhibit GABAergic neurotransmission, but perhaps counterintuitively this action would appear to have a neuroprotective effect (Chen et al. 2014). It is also noteworthy that this would appear to be contrary to the effects of systemic inflammation on the GABA system, leading to its activation, which is considered to be one source of apparently idiopathic chronic pain (Jang et al. 2017). This may be a mechanism underpinning the presence of the chronic intractable pain reported by many CFS patients (Nijs et al. 2012). In this context it is also worth noting that microglial activation, most notably in the basal ganglia, is also a well-documented cause of ‘unexplained’ chronic pain (Jeon et al. 2017).
Systemic LPS can enter the CNS via regions of BBB permeability such as the circumventricular organs and area postrema, in much the same way as PICs, and engage TLR4 receptors on microglia leading to their activation (Hines et al. 2013; Konsman et al. 2002; Sandiego et al. 2015). The resulting neurocognitive dysfunction has much the same origins as microglial activation induced by PICs but there is some evidence to suggest that the adverse effects on adult neurogenesis, memory deposition and recall, synaptic plasticity and long-term potentiation following elevated systemic LPS is primarily mediated by elevated IL-1β in the hippocampus (Abareshi et al. 2016; Li et al. 2017; Nolan et al. 2005).
Chronic neuroinflammation as the cause of neurocognitive and neuroimaging abnormalities
It should also be stressed at this junction that microglia and astrocytes play indispensable roles in maintaining CNS homeostasis in areas such as synaptic plasticity and long-term potentiation, which are essential for the deposition and retrieval of memory representations, together with oxygen and nutrient delivery to neurones; thus dysregulated activity of these glial cells is detrimental to cognitive function (Sofroniew and Vinters 2010; Xavier et al. 2014). Hence the development of functional gliopathology in combination with disturbances to neurotransmission following the activation of microglia and astrocytes discussed above could explain, at least in part, the multiple lines of evidence demonstrating cognitive dysfunction in patients with CFS.
This would also seem to be true of neuroimaging abnormalities seen in CFS patients as there is direct evidence that neuroinflammation resulting from microglial activation disrupts resting state functional connectivity (Colasanti et al. 2016) and there is copious evidence that neuroinflammation is a cause of structural damage and of GM and WM atrophy in a range of neurological and medical conditions (Calabrese et al. 2015; Chen et al. 2015; Cheriyan et al. 2012; Chiang et al. 2017; Raj et al. 2017; Tóth et al. 2017; Zhang et al. 2016).
The existence of chronic ONS in the CNS following the activation of microglia and astrocytes subsequent to the existence of chronic peripheral inflammation may also explain the development of cerebral hypoperfusion in CFS patients. Briefly, high levels of NO and ROS result in oxidative damage to lipids, proteins and DNA in the endothelial cells of the BBB resulting in a pattern of escalating damage to such cells and a concomitant loss of the cytoprotective effects of NO normally derived from endothelial nitric oxide synthase (eNOS) (Lucas et al. 2015; Morris and Maes 2014). This is the result of oxidative inactivation of tetrahydrobiopterin (BH4), which is one of the enzyme’s essential cofactors, and changes in levels of arginine and calcium ions (Burghardt et al. 2013; Mitchell et al. 2007; Montezano and Touyz 2012). It should also be noted at this juncture that chronic peripheral inflammation can also impair endothelial eNOS function (Burghardt et al. 2013).
The mechanism underpinning depleted BH4 levels under such conditions involves ROS-induced oxidation of BH4 to dihydrobiopterin (BH2), subsequently reducing levels of the former molecule in the endothelium of the BBB (Najjar et al. 2013). The subsequent decrease in the BH4 to BH2 ratio results in the inhibition of eNOS while simultaneously uncoupling arginine as its substrate thereby enabling engagement with environmental oxygen and increased production of superoxide ions (Bouloumie et al. 1999; Moens and Kass 2006; Najjar et al. 2013). The resultant combination of superoxide ions with NO results in further increases in levels of ONOO−, thereby inducing increased oxidation of BH4 to BH2, further decreasing the activity of eNOS in an escalating positive feedback loop (Chen et al. 2010; Szabó et al. 2007).
Crucially, reduced eNOS activity can deplete endothelial NO levels, ultimately resulting in significantly impaired CBF (Najjar et al. 2013; Toda and Okamura 2012). The development of this phenomenon also appears to be associated with impaired vasodilation which also stems from impaired neurovascular eNOS-dependent synthesis of NO (Li et al. 2016a; Liu et al. 2016; Najjar et al. 2013). Furthermore, persistent cerebral hypoperfusion can compromise endothelial mitochondrial respiration further increasing the formation of ROS in BBB endothelial cells (Aliev et al. 2010, 2014; Liu and Zhang 2012), which in turn promotes increased eNOS uncoupling, further lowering endothelial NO levels, and a pattern of incrementally decreasing cerebral perfusion in a positive feedback loop (Antoniades 2006; Chen et al. 2010; Lavoie et al. 2010).
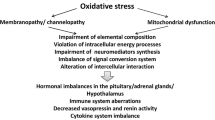
Importantly, the existence of positive feedback loops in the brain and periphery, such as those discussed above, can lead to a pattern of increasing I&ONS, which can trigger a state of metabolic and immune downregulation potentially accounting for a range of apparently conflicting data reported by researchers investigating these domains even in participants afforded a diagnosis of CFS according to the international consensus criteria. These processes form the focus of the remainder of this paper and are discussed below.
Increasing I&ONS and the switch to immune and metabolic downregulation
Advent of hypernitrosylation
Reversible protein S-nitrosylation, denitrosylation and transnitosylation of protein cysteine thiols effects the vast bulk of NO cellular signalling and enables the homeostatic regulation of virtually every dimension of redox-dependent protein signalling, largely determining protein function, stability and trafficking (Banerjee 2012; Hill and Bhatnagar 2012; Paulsen and Carroll 2010; Winterbourn and Hampton 2008).
From the perspective of this paper, the key point to stress is that this NO-induced post-translational modification plays an indispensable role in maintaining cellular homeostasis in the face of increasing levels of oxidative stress (Gorelenkova Miller and Mieyal 2015; Okamoto and Lipton 2015). In such an environment, moderate increases in levels of ROS and NO lead to a defensive pattern of increased S-nitrosylation of crucial structural and functional proteins as a shield against irreversible oxidation of cysteine thiols and subsequent prolonged or even permanent changes in their secondary and tertiary conformation leading to inactivity and/or immunogenicity (Kohr et al. 2014; Penna et al. 2014; Sun and Murphy 2010; Sun et al. 2006). However, further increases in O&NS lead to impaired activity of denitrosylases such as the thioredoxin system, S-nitrosogluthathione reductase, protein disulphide isomerase, superoxide dismutase and glutathione peroxidase, which maintain the reversibility of S-nitrosylation leading to a state of protracted or even irreversible nitrosylation which has been described as hypernitrosylation (Wu et al. 2010; Wu et al. 2011) (reviewed (Morris et al. 2017c)).
A state of increased nitrosylation in CFS is indicated by findings that IgM responses to NO-tryptophan, NO-tyrosine, NO-albumin and NO-cysteinyl are increased in CFS (Maes et al. 2006). Interestingly, in CFS, increased bacterial translocation is associated with indicants of increased nitrosylation (Maes and Leunis 2014). A wider discussion of the potential role of NO and ONOO− in the pathogenesis and pathophysiology of CFS may be found in a recent review by Monro and Puri (2018). This is of importance as the development of ‘hypernitrosylation’ can provoke a switch from an inflammatory environment with excessive activation of immune pathways to an environment of hypo-inflammation, impaired energy production and metabolic downregulation, which may be exacerbated in individuals with high levels of translocated LPS as high systemic concentrations of this antigen may result in the same ultimate endpoints (Morris et al. 2016b, c). This will form the theme of the remainder of this paper with initial impairment in oxidative phosphorylation and mitochondrial dynamics considered immediately below.
Hypernitrosylation and impaired mitochondrial performance
There is a considerable and accumulating body of data demonstrating that S-nitrosylation of key mitochondrial enzymes and structural proteins plays an indispensable role in the redox-based regulation of mitochondrial respiration and other aspects of energy production (Doulias et al. 2013; Mailloux et al. 2014). In sum, readily reversible S-nitrosylation negatively regulates the function of a myriad of proteins involved in oxidative phosphorylation, the tricarboxylic cycle, gluconeogenesis, glycolysis, generation of mitochondrial ROS, mitochondrial permeability transition, as well as apoptosis (Doulias et al. 2013; Mailloux et al. 2014). Hence hypernitrosylation has the capacity potentially to impair cellular energy generation and, in particular, to lead to inhibitory nitrosylation of crucial cysteine thiols of enzymes in the electron transport chain (ETC) such as complex I (Drose et al. 2014; Murray et al. 2012; Piantadosi 2012), cytochrome c oxidase (complex IV) and, to a lesser extent, complex II (Sarti et al. 2012a; Zhang et al. 2005). Unsurprisingly, such inhibition impairs oxidative phosphorylation and the production of ATP, and depletes levels of mitochondrial glutathione owing to increased production of ROS by a dysfunctional ETC (Sarti et al. 2000, 2003a, b, 2012b; Zhang et al. 2005). There are also data demonstrating that prolonged inhibition of cytochrome c oxidase increases ATP production by glycolysis at least in some cell types as an attempt to mitigate against cell death via apoptosis or necrosis (Almeida et al. 2001; Bolanos et al. 2010; Burwell et al. 2006; Shiva et al. 2007; Sun et al. 2007).
In addition, prolonged nitrosylation of the mitochondrial regulatory proteins PINK-1, PARKIN and DRP-1 compromises mitochondrial dynamics as a result of impaired mitophagy, increased degeneration and increased fission (Nakamura et al. 2010; Oh et al. 2017; Ozawa et al. 2013; Reddy et al. 2011). This may be relevant from the perspective of activity intolerance reported by many CFS patients as intact mitochondrial dynamics enables the adaptation of an individual to continuous or incremental exercise regimes (Trewin et al. 2018; Yan et al. 2012).
Hypernitrosylation and the development of immune suppression and cellular hibernation
Prolonged or intractable S-nitrosylation characteristic of hypernitrosylation may lead to changes in the activity of proteins and signalling pathways regulating inflammation and energy production, leading to a state of hypo-inflammation and metabolic downregulation. For example, hypernitrosylation of a crucial cysteine residue of p50 silences NF-κB-dependent gene transcription via several different mechanisms (Bogdan 2001; DelaTorre et al. 1997, 1998, 1999; Kelleher et al. 2007). In addition, hypernitrosylation of cytosolic enzyme inhibitory kappaB kinase beta impairs its levels of phosphorylation (Reynaert et al. 2004) leading to diminished proteasomal degradation of inhibitory kappaB which maintains NF-κB in an inactive state (Hess et al. 2005; Marshall et al. 2004; Reynaert et al. 2004). In addition, hypernitrosylation can further contribute to the development of immune suppression or an hypo-inflammatory state by the downregulation of TLR signalling via inhibition of MyD88 thereby inhibiting the immune response to acute pathogen invasion (Into et al. 2008). Increased NO levels may also upregulate IRAK-M mRNA and protein expression of interleukin receptor associated kinase-M (IRAK-M), which is a key negative regulator of TLR signalling although there is no compelling data indicating that this process is enabled by S-nitrosylation either directly or indirectly (del Fresno et al. 2004; Gonzalez-Leon et al. 2006). These pathways are illustrated in Fig. 1.
Acute stimulation of TLR4 leads to the recruitment of MyD88, MyD88-adaptor-like (MAL), IL-1 receptor-associated kinase-4 (IRAK-4), toll/IL-1 receptor (TIR)-domain-containing adaptor-inducing IFNβ (TRIF) and TRIF-related adaptor molecule (TRAM), ultimately provoking the initiation of signalling cascades that converge to activate NFκB, MAPKs and IFN response factors (IRFs) with the subsequent production of inflammatory mediators such as type 1 interferons and PICs. However, in a state of endotoxin tolerance the TLR4 response is reprogrammed via the upregulation of the inhibitory proteins SHIP1, suppressor of cytokine signalling 1 (SOCS1) and the pseudokinase IRAK-M leading to the production of IL-10 and TGF-β1 leading to a downregulated and anti-inflammatory immune response
Prolonged S-nitrosylation (see Fig. 2) can also lead to profound metabolic changes via upregulation of the protein subunit hypoxia-inducible factor-1α (HIF-1α), through upregulation of HIF1A and/or stabilisation of this subunit, under non-hypoxic conditions (Kasuno et al. 2004; Li et al. 2007; Yasinska and Sumbayev 2003) and activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mTOR signalling (Gupta et al. 2017; Kwak et al. 2010; Lopez-Rivera et al. 2014; Numajiri et al. 2011). There is also evidence that mTOR is directly activated as a result of inhibitory S-nitrosylation of tuberous sclerosis complex 2 (TSC2), an inhibitor of mTOR (Lopez-Rivera et al. 2014) and nitrosylation-mediated activation of Ras, a small GTPase which is a positive regulator of mTOR (Lee and Choy 2013). Prolonged nitrosylation may also provoke changes in metabolic pathways via the upregulation of GSK-3 (Morris et al. 2017c). Furthermore, activated GSK-3 and PI3K/Akt signalling in tandem or separately can provoke metabolic and bioenergetic dysregulation by inhibiting the enzyme 5′-adenosine monophosphate (AMP)-activated protein kinase (AMPK) (Park et al. 2014; Suzuki et al. 2013).
Proteins undergo reversible S-nitrosylation and denitrosylation initiated by the covalent addition and release of NO probably derived from N2O3 in a hydrophobic environment. Denitrosylation may be mediated by several molecular players such as GSNOR (S-nitrosoglutathione (GSNO) reductase) and the thioredoxin system. Transnitrosylation is another major route mediating protein nitrosylation. Transnitrosylation of GSH leading to the formation of native protein and GSNO is a major element enabling this process. Reformation of GSH by NADPH involves NADH-dependent reduction by GSNOR to generate GSH. The GSH and thioredoxin systems are progressively inactivated in an environment of increasing chronic ONS leading to the inhibition of denitrosylation and transnitrosylation and a state of protein ‘hypernitrosylation’
These observations may be directly relevant as far as the pathogenesis of CFS is concerned as activation of HIF-1α, PI3K/Akt/mTOR and GSK-3 signalling pathways and inhibition of AMPK, in the context of decreased canonical NF-κB activation, are major elements in the development of a phenomenon described as endotoxin tolerance which involves profound systemic immune and metabolic downregulation, with the latter phenomenon involving sequential shifts in energy production via glycolysis and oxidation of fatty acids, following prolonged TLR upregulation in macrophages and monocytes (Liu et al. 2011a, 2012). This phenomenon is mediated, at least in part, by epigenetic reprogramming ‘masterminded’ by sirtuin 1 (SIRT1) (Liu et al. 2011a, 2012) and in this context decreased levels of canonical NF-κB signalling induced by persistent nitrosylation would be expected to activate SIRT1 (Kauppinen et al. 2013; Vaziri et al. 2001; Yang et al. 2012). This is important as SIRT1 nitrosylation in the context of high levels of classical NF-κB signalling secondary to chronically elevated I&ONS or the response to acute pathogen invasion leads to its inactivation (Kalous et al. 2016; Nakazawa et al. 2017; Shinozaki et al. 2014).
Before moving on to consider the mechanisms underpinning the development of endotoxin tolerance, it is important to note that this phenomenon resolves within days and sometimes weeks in the context of DAMP- or pathogen-associated molecular pattern-mediated engagement of PRRs, but in the context of the same result mediated by chronically elevated I&ONS and hypernitrosylation a state similar to endotoxin tolerance could be protracted or even permanent in the absence of ameliorative interventions.
Mechanisms underpinning metabolic downregulation in endotoxin tolerance
During the initial inflammatory phase following TLR activation, NF-κB upregulates the Akt-mTOR-HIF-1α pathway, leading to a surge in ATP production via aerobic glycolysis (Cheng et al. 2014a; Srivastava and Mannam 2015; van Uden et al. 2008). Such upregulation leads to inhibition of the tricarboxylic acid (TCA) cycle and increased production of mitochondrial ROS, leading to a fall in mitochondrial ATP production, mitochondrial structural damage, increased mitophagy and a switch in cytochrome oxidase subunits designed to increase the efficiency of the ETC (Cheng et al. 2014a; Semenza 2011; Zhong et al. 2010). Inhibition of the TCA cycle and oxidative phosphorylation is mediated by increase in the transcription of pyruvate dehydrogenase kinase with the subsequent inhibition of pyruvate dehydrogenase and conversion of pyruvate into acetyl coenzyme A (acetyl-CoA). This inhibition is accompanied by a concomitant HIF-1α-mediated increase in the conversion of pyruvate to lactate with the resultant production of ATP and NAD+ by aerobic glycolysis (Kim et al. 2006).
The continuation of ATP production and NAD+ by this route is enabled by HIF-1α-induced upregulation of lactate dehydrogenase (Semenza et al. 1996). The switch from ATP generation from oxidative phosphorylation instigated by the upregulation of the transcription factor is further enabled by increased expression of glucose transporters and glycolytic enzymes (Hanahan and Weinberg 2011; Semenza 2003). The importance of Akt-mTOR-HIF-1α signalling in the development of endotoxin tolerance is emphasised by data demonstrating that inhibition of this pathway prevents the development of immune and metabolic downregulation in macrophages and monocytes (Cheng et al. 2014a).
However, this rapid burst of ATP production and concomitant increases in NADH production enabled by activation of the of Akt-mTOR-HIF-1α signalling system is a short-lived phenomenon and the subsequent fall in ATP and NADPH generation leads to a relative rise in AMP and NAD+ (Adriouch et al. 2007; Haag et al. 2007), leading to the activation of AMPK (Gómez et al. 2015) and the sequential activation of the NAD+-sensitive SIRT family members sirtuins 1, 6 and 3 (Liu et al. 2011a, 2012, 2015a). Once activated, these SIRTs play the dominant role in the development of a hypo-inflammatory state and an environment of metabolic downregulation and reductions in glycolysis and TCA-induced oxidative phosphorylation characterised by mitochondrial ATP production via fatty acid oxidation (Gómez et al. 2017; Vachharajani et al. 2016). This adaptive low-energy state is often described as cellular ‘hibernation’ (Levy et al. 2005; Liu et al. 2011a; Singer 2008) (reviewed (Singer 2017)) and it is important to stress that this phenomenon is not limited to macrophages and monocytes but also takes place in hepatocytes and striated muscle cells (Carré and Singer 2008; McCall et al. 2011; Singer 2007, 2008).
Once activated, SIRT1 upregulates the transcription of peroxisome proliferator-activated receptor γ coactivator-1 alpha with a subsequent and concomitant increase in mitochondrial biogenesis and respiration mediated by fatty acid oxidation. The mechanisms underpinning this process include increased cellular uptake of fatty acids via the CD36 membrane transporter and increased transfer of these molecules into mitochondria via upregulation of several enzymes including palmitoyltransferase I, which governs the rate of fatty acid oxidation (Liu et al. 2012; Vachharajani et al. 2014; Wanders et al. 2010) (reviewed (Qu et al. 2016)). SIRT1 also exerts a range of protective effects on mitochondria aimed at maximising energy generation and fostering cellular and organelle survival. These effects include: upregulating cellular anti-oxidant defences, via activation of nuclear factor (erythroid-derived 2)-like 2; increasing mitophagy to remove damaged mitochondria; and maintaining mitochondrial membrane potential, thereby inhibiting the development of mitochondrial permeability transition pore opening (Price et al. 2012; Song et al. 2017).
SIRT1-induced upregulation of SIRT6 also encourages the switch from ATP generation via aerobic glycolysis to ATP generation via fatty acid oxidation, via direct and indirect inhibition of glycolysis, increased fatty acid oxidation and a range of effects broadly encouraging mitochondrial survival in a low-energy state (Cheng et al. 2016; Sebastian et al. 2012). Direct inhibitory effects of SIRT6 on glycolysis include downregulation of glucose transporter 1 and inhibition of lactate production (Elhanati et al. 2016; Long et al. 2017), while indirect effects stem from inhibition of HIF-1α (Sebastian et al. 2012). SIRT6 elevation also preserves mitochondrial membrane potential, preventing mitochondrial permeability transition pore (mPTP) opening in a similar manner to SIRT1 (Cheng et al. 2016).
Upregulation of SIRT3, an NAD+-dependent mitochondrial protein, also deacetylates and activates mitochondrial enzymes involved in fatty acid β-oxidation, amino acid metabolism, the TCA cycle, the ETC and antioxidant defences leading to increased mitochondrial ATP production (Ahn et al. 2008; Ansari et al. 2017). In addition, increased SIRT3 activity inhibits mitochondrial ROS production and the activity of several components of the mPTP, thereby encouraging mitochondrial survival (Kincaid and Bossy-Wetzel 2013; Tseng et al. 2013) (see Fig. 3).
S-Nitrosylation leads to the inhibition of Complex 1, Complex IV, the F1F0ATPase (Complex V) and possibly Complex II leading to the reduction of ATP production and a decrease in ROS production with a subsequent increase in production of ATP via aerobic glycolysis. S-Nitrosylation can also compromise mitochondrial function while increasing the survival of the organelle by inhibiting uptake of calcium ions and reduction of cytosolic calcium ions via inhibition of SERCA, further reducing mitochondrial ATP and ROS production. S-Nitrosylation also inhibits key enzymes of the TCA cycle, such as aconitase and α-ketoglutarate dehydrogenase, and regulates those involved in fatty acid metabolism, thus further inhibiting oxidative phosphorylation and stimulating aerobic glycolysis and mitochondrial fatty acid oxidation
It is also noteworthy that the sequential activation of these SIRTs corresponds with the inactivation of AMPK (Jiang et al. 2014; Liu et al. 2015b). Unsurprisingly, the causes of this phenomenon have been the subject of intensive research, and while the process may be multifactorial in origin, the weight of data implicates inhibition by activated GSK-3 (Park et al. 2014; Suzuki et al. 2013). The upregulation of GSK-3 in turn appears to result from the termination of the inhibitory influence of Akt/mTOR signalling by the upregulation of SIRT1 and SIRT6 and the termination of ATP production by aerobic glycolysis (Frost and Lang 2011; Ghosh et al. 2010; Hermida et al. 2017; Pillai et al. 2014). Another contributing factor underpinning the inactivation of AMPK might be the relative increase in mitochondrial ATP production and a reduction in mitochondrial ROS production as a result of a switch to fatty acid β-oxidation from aerobic glycolysis (reviewed (Nsiah-Sefaa and McKenzie 2016)) would be below the cellular AMP and ROS thresholds expected to trigger the activation of the enzyme (Morris et al. 2017d).
SIRT1 activation and subsequent immune downregulation
Once activated, SIRT1 also plays a pivotal role in the development of a hypo-inflammatory immune response. In essence, this is achieved by inhibiting the transcription of IL-1β, TNF-α and other proinflammatory genes and suppressing inflammatory responses by deacetylating and inhibiting the incumbent p65 component of the NF-κB complex while inducing the expression of RelB (Kauppinen et al. 2013; Liu et al. 2011a; Yang et al. 2012) as well as causing gene-specific regulation as histone modifiers (Foster et al. 2007; Gazzar et al. 2008; Liu et al. 2015a; McCall et al. 2010; Yoza et al. 2006).
From a more mechanistic perspective, SIRT1 deacetylates histone protein H1K27 and lysine 310 (Lys310) of RelA/p65 and its continued binding recruits RelB to promoters of target anti-inflammatory genes (Liu et al. 2011a; Millet et al. 2013). In this instance RelB acts as a dual-function transcription factor; it increases silent facultative chromatin at promoters of pro-inflammatory genes via direct interaction with histone H3 lysine 9 methyltransferase G9a, heterochromatin protein 1, assembly of high mobility group box 1 and DNA CpG methylation; (Chen et al. 2009; Gazzar et al. 2009; Millet et al. 2013; Yoza and McCall 2011) and active euchromatin at anti-inflammatory genes via association with the NF-κB subunit p50 and the resultant transcription of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (McCall et al. 2010). In addition, SIRT6 also deacetylates RelA/p65 and histone H3K9Ac to exert additional anti-inflammatory activity (Gil et al. 2013; Kawahara et al. 2009).
However, the activation of SIRT 1 and SIRT 6 are not the only elements involved in the immune downregulation characteristic of endotoxin tolerance as miRNA activity, soluble factors such as IL-10 and TGF-β1 and the concomitant downregulation of proteins enabling TLR signalling and upregulation of proteins such as SH2 (Src Homology 2)-containing inositol phosphatase (SHIP) and IRAK-M, known to inhibit TLR signalling, all play an indispensable role (reviewed (Wisnik et al. 2017; Fu et al. 2012)). Crucially, these processes may begin with the activation of indoleamine 2,3-dioxygenase (IDO) which is activated in an environment of excessive I&OS (Morris et al. 2016a), as discussed below.
I&ONS and the development of endotoxin tolerance via IDO upregulation
Chronic I&ONS can also provoke the development of endotoxin tolerance by inducing the transcriptional activation of IDO (Kim et al. 2015c; Wichers and Maes 2004) leading to upregulation of the kynurenine pathway, aryl hydrocarbon receptor (AhR) activity and increased levels of TGF-β1 (Bessede et al. 2014; Wirthgen and Hoeflich 2015) and IL-10 (Alexeev et al. 2016; Lanis et al. 2017) via well documented mechanisms (reviewed (Wirthgen and Hoeflich 2015)).
The upregulation of AhR activity is of interest given data presented in the previous section as increased activity of this cytosolic transcription factor leads to upregulation of RelB and non-canonical NF-κB signalling (Salazar et al. 2017; Vogel et al. 2013). Mechanistically, these effects appear to be mediated by transcriptional upregulation of RelB (de Souza et al. 2014; Thatcher et al. 2007) and subsequent physical engagement between RelB and AhR to produce dimers capable of modulating the expression of NF-κB-sensitive genes (Vogel et al. 2008). AhR-upregulated RelB also stimulates and maintains the transcription of miR-146a (Zago et al. 2014, 2017). This is of importance as miR-146a is a dominant player in the development and maintenance of the hypo-inflammatory environment characteristic of endotoxin tolerance (Banerjee et al. 2013; Nahid et al. 2009). Mechanistically, this inhibitory effect is enabled by suppressing TLR signalling pathways by reducing the translation of TNF receptor associated factor 6 (TRAF6), interleukin-1 receptor-associated kinase 1 (IRAK1), IRAK2 and interferon regulatory factor 3 (IRF3), which are positive adaptor kinases of MyD88-mediated signalling and hence their inactivation results in reduced activity of both NF-κB and IRF3 (Nahid et al. 2011) (reviewed (Testa et al. 2017)).
The upregulation of TGF-β1 also results in upregulated non-canonical NF-κB signalling (Pallotta et al. 2011; Shi and Massague 2003). Increased activation of this cytokine also upregulates pseudokinase IRAK-M (Pan et al. 2010; Srivastav et al. 2015; Standiford et al. 2011). This is significant because IRAK-M would appear to be the ‘master regulator’ of the TLR pathway suppression characteristic of the state of endotoxin tolerance in PMBCs (del Fresno et al. 2007; Escoll et al. 2003; Stiehm et al. 2013; van’t Veer et al. 2007; Wiersinga et al. 2009). Indeed, the weight of evidence suggests that increased activity of this enzyme alone is sufficient to maintain an LPS-induced hypo-inflammatory state in human macrophages and monocytes (van’t Veer et al. 2007). This is unsurprising given that this molecule can inhibit TLR signalling at multiple levels. TGF-β1 has been established as an indispensable element in the development of endotoxin tolerance-associated SHIP upregulation (Sly et al. 2004; Yang et al. 2015). This may be of particular relevance from the perspective of a putative explanatory model of CFS aetiology as elevated levels of this cytokine in PMBCs and whole blood are a common finding in patients diagnosed according to narrow international consensus criteria and correlate with the severity of a range of symptoms (Blundell et al. 2015; Wyller et al. 2017). Once again, it is noteworthy that this phenomenon is not observed in patients diagnosed according to broader schema which are not internationally recognised such as the ‘alternative CDC criteria’ (Clark et al. 2017).
Upregulated IL-10 also exerts negative effects on TLR signalling by increasing the ubiquination and proteasome-mediated degradation of a range of MyD88-dependent signalling effector molecules such as IRAK-4 and TRAF6 ultimately resulting in reduced phosphorylation and activity of inhibitor of kappa B kinase (IKK), p38 and JNK (Chang et al. 2009). IL-10 is produced by monocytes, macrophages, Tregs and Th2-polarised T cells in a state of endotoxin tolerance, and suppresses the CD8 T and CD4 Th1 type cell response making an indispensable contribution to the development of an anti-inflammatory environment (Jiang and Chess 2006; Littman and Rudensky 2010). The indispensable contribution of IL-10 to the development of endotoxin tolerance (Liu et al. 2011b; Quinn et al. 2012) is of importance from the perspective of this paper as the upregulation of this cytokine is a common observation in CFS patients (Roerink et al. 2017; Wong et al. 2015).
It should be noted that once activated, IDO activity can be maintained by two positive feedback mechanisms. First, TGF-β can target its cellular receptor leading to the upregulation of NF-κB-RelB signalling leading to further transcription of IDO (Pallotta et al. 2011; Shi and Massague 2003). Second, IDO-activated AhRs can in turn upregulate the transcription of IDO1 (the gene that encodes IDO) via genomic and non-genomic routes (Li et al. 2016b; Litzenburger et al. 2014). Hence once activated, IDO upregulation could be protracted or even chronic.
In addition, there is evidence obtained from human studies that chronic or intermittent translocation of LPS into the systemic circulation can induce a state of tolerance and alternative activation in macrophages and monocytes characteristic of endotoxin tolerance via the activation of IDO, kynurenine and the AhR (Banerjee et al. 2013; del Campo et al. 2011; del Fresno et al. 2008; Pena et al. 2011; Wisnik et al. 2017). Given the existence of LPS translocation in CFS, this mechanism could also contribute to the development of a chronic state resembling endotoxin tolerance.
The importance of IDO activation in the development of endotoxin tolerance is further emphasised by data confirming that interactions between the AhR, kynurenine and TGF-β1 are responsible for the polarisation of activated naïve T cells into the Treg phenotype by the presentation of antigen by tolerogenic antigen-presenting cells (Gandhi et al. 2010; Mezrich et al. 2010). Such phenotypic presentations are considered below.
IDO2 is a homologue of IDO (also known as IDO1), being an immunomodulatory enzyme which catalyses L-trytophan; like IDO1, IDO2 is also located on chromosome 8 in humans but IDO2 is not as widely expressed as IDO1 and IDO2 has a distinct signalling role (Metz et al. 2007; Cha et al. 2018). B cell IDO2 expression has recently been identified as being an essential mediator of autoreactive B and T cells in autoimmune responses (Merlo and Mandik-Nayak 2016; Merlo et al. 2016, 2017). It seems likely, therefore, that IDO2 may be found to play an important role in ME/CFS.
State of leukocytes in endotoxin tolerance
Neutrophils
There are not a great deal of data regarding these immune cells in a state of endotoxin tolerance but the evidence in existence is typical of that would be expected if such cells were in a state of immune downregulation. For example, Parker and fellow workers reported a downregulation of TLR4 receptors, high levels of IL-8 secretion and impaired oxidative burst in neutrophils in endotoxin tolerance (Parker et al. 2005). Impaired rolling, endothelial cell adhesion and migration to sites of infection have also been reported (Alves-Filho et al. 2008; Ogawa et al. 2003). However, there appears to be considerable variation in the migration of neutrophils in this condition as another research team has reported that this ability may be significantly increased (Ariga et al. 2014).
Monocytes and macrophages
Stimulated macrophages and DCs in a state of endotoxin tolerance display M2 polarisation as evidenced by decreased production of PICs such as IL-12, IL-6 and TNF-α and an increased production of IL-10 and TGF-β1 (Albrecht et al. 2008; del Fresno et al. 2009). The magnitude of such immune downregulation is thrown into stark relief by data demonstrating that levels of TNF-α, IL-1β and IL-6 in activated monocytes in this condition are typically less than 10–20% of levels in stimulated monocytes extracted from healthy controls (Boomer et al. 2014; Rigato and Salomao 2003; Sinistro et al. 2008).
Macrophages and DCs in a state of endotoxin tolerance also display impaired antigen presentation owing to downregulated major histocompatibility complex (MHC) class II molecules such as human leukocyte antigens – antigen D related (HLA-DRs) and class II transactivator and the costimulatory molecule CD86 which may be caused, at least in part, by elevated levels of TGF-β and IL-10 (Biswas and Lopez-Collazo 2009).
DCs in particular appear to be immature and their activation appears to be impaired as evidenced by reduced production of chemokine (C-C motif) ligand 3 (CCL3) and CCL5 (Albrecht et al. 2008), reduced levels of CD80 and CD86 (Cohen et al. 2004) and evidence of reduced numbers (Ishiyama et al. 2006).
Curiously, while there is evidence of reduced numbers of DCs as a whole (Ishiyama et al. 2006), it would appear that the relative proportion of myeloid DCs increases leading to a higher level of antigenic stimulation overall, in turn leading to the differentiation of naïve T cells along the Th2 pathway but producing T lymphocytes with a characteristically reduced expression of IL-2 and interferon gamma (IFNγ) but normal levels of IL-4 and IL-5 (Ishiyama et al. 2006; Lauw et al. 2000).
These tolerogenic DCs also play a major part in inducing CD4 and CD8 T cell anergy, inhibition of T cell effector and memory responses and inducing the activation and generation of Tregs, which are all characteristic of endotoxin tolerance (Domogalla et al. 2017; Raker et al. 2015).
T cell characteristics in endotoxin tolerance
A protracted state of endotoxin tolerance is characterised by CD4 T cell exhaustion or anergy, impaired CD8 T cell proliferation and an absolute rise in the numbers and suppressor function of Tregs (Cabrera-Perez et al. 2014; Cao et al. 2015; Strother et al. 2016). Anergic or exhausted T cells are unresponsive to antigenic stimulation and express high levels of inhibitory receptors on their surfaces such as CD69, programmed death receptor-1 (PD-1), CD25, IL-7R and T cell membrane protein-3 (Boomer et al. 2011) (reviewed (Boomer et al. 2014)). While upregulation of each receptor plays a part in maintaining T cell anergy, there is accumulating evidence that PD-1 and its ligands play the dominant role and that immunotherapy directed at this receptor complex can restore T cell function in vivo (Araki et al. 2013; Lee et al. 2015). Such T cells in this hypo-inflammatory state also display evidence of repressive histone methylation in T-bet (a T-box transcription factor), GATA-3 and retinoic acid receptor (RAR)-related orphan receptor gamma, which would go some way to explaining their unresponsiveness to antigenic stimulation (Pachot et al. 2005) (reviewed (Carson and Kunkel 2017)). It is noteworthy that the pattern of T cell anergy is also seen in patients with chronic viral infections because of unrelenting antigenic stimulation (Yi et al. 2010). This level of CD4 T cell dysfunction is also associated with reactivation of latent herpes viruses which can add to the antigenic milieu and, combined with translocated LPS, could afford another avenue maintaining a persistent a hypo-inflammatory environment (Laing et al. 2012; Limaye et al. 2008; Ouwendijk et al. 2013).
PD-1 upregulation appears to be the dominant player in the development and maintenance of CD8 T cell anergy and loss of CD8 T cell numbers seen in an environment of endotoxin tolerance although the underlying mechanism may be different in this case and such upregulation appears to be driven by elevated levels of TGF-β1 (Baas et al. 2016; Danahy et al. 2016). The level of proliferative and functional impairment in memory CD8 T cells extends to all modes of activation, with decreased antigen-dependent sensitivity and an impaired capacity to detect the presence of inflammatory mediators in the immediate microenvironment being repeatedly reported, which dramatically reduces their effectiveness in dealing with reinvading pathogens (Duong et al. 2014).
The increased numbers and suppressive capacity of Tregs seen during the development of endotoxin tolerance also play a major role in the development of T cell anergy either via a contact-dependent mechanism or via increased secretion of TGF-β and IL-10 (Cao et al. 2015; Jiang et al. 2012). Finally, and perhaps predictably, the development of endotoxin tolerance is associated with decreased numbers and function of NK cells, with decreased IFNγ production by CD56 NK cells being the most commonly reported functional impairment (Chiche et al. 2011; Forel et al. 2012; Pan et al. 2014).
Can immune and metabolic abnormalities be explained by endotoxin tolerance?
Metabolic downregulation
The data presented above relating to metabolic downregulation are potentially important observations from the perspective of CFS pathogenesis and/or pathophysiology as they could explain apparently diverse observations in PMBCs such as increased energy production by glycolysis (Lawson et al. 2016), impaired pyruvate dehydrogenase function (Fluge et al. 2016), lower mitochondrial ATP production and disordered lipid metabolism compared with healthy controls (Germain et al. 2017; Tomas et al. 2017) and a cellular state of apparent metabolic hibernation (Naviaux et al. 2016). There is also evidence that NAD+ levels may be elevated in CFS patients (Ciregia et al. 2016; Mikirova et al. 2012; Naviaux et al. 2016). Furthermore, the dysfunction of the TCA cycle in CFS patients reported by (Yamano et al. 2016), inhibition of glycolysis and reduced levels of pyruvate reported by (Armstrong et al. 2015) and inactivated AMPK together with subnormal levels of IL-6 in the striated muscles of CFS patients reported by (Brown et al. 2015) are all abnormalities consistent with a pattern of metabolic downregulation associated with the cellular hibernation associated with endotoxin tolerance. It is also noteworthy that the impaired level of oxidative phosphorylation in PMBCs of CFS patients recently reported by (Tomas et al. 2017) is consistent with mitochondrial ATP production via the β-oxidation of fatty acids. Furthermore, the recently published new model for chronic disease pathogenesis and treatment by Naviaux (2018) is germane; in this model, the pathophysiology of chronic disease is re-framed in terms of metabokine and mitochondrial signalling abnormalities.
There is also evidence that AMPK becomes inactivated during a state of endotoxin tolerance, probably as a result of elevated GSK-3 activity (Jiang et al. 2014; Liu et al. 2015b). This may be significant from the perspective of exercise intolerance seen in ME/CFS as this kinase is responsible for sensing and enabling increased ATP production and maintaining metabolic homeostasis in striated muscle cells in response to increasing demands for energy during exercise (Morales-Alamo and Calbet 2016; Trewin et al. 2018). AMPK signalling also plays an indispensable role in stimulating increased levels of fatty acid oxidation needed to foster the recovery of skeletal muscle function and dynamics in the aftermath of exercise (Egan and Zierath 2013). In the context of a putative explanatory model of the causes of the CFS, it is noteworthy that AMPK appears to be inactive in the striated muscle of at least some patients afforded this diagnosis (Brown et al. 2015). Hence the phenomenon of endotoxin tolerance could go some way to explaining the muscle dysfunction, exercise intolerance and prolonged recovery time characteristic of CFS (reviewed (Gerwyn and Maes 2017)). In addition, the fact that increased levels of NO and ROS generated by mitochondria during incremental endurance training can lead to oxidative inactivation of AMPK (Morales-Alamo and Calbet 2016) could explain the relative failure of long-term incremental training regimes to produce any clinically significant benefits in the short or long term in recent trials involving CFS patients initially diagnosed according to the widest existing criteria (White et al. 2011).
Immune phenotype associated with endotoxin tolerance
The concept of endotoxin tolerance would go some way to providing an explanation of the apparently contradictory patterns of pro- and anti-inflammatory cytokine production and Th1 and Th2 biases which are common in the literature (Brenu et al. 2011; Hornig et al. 2017; Hornig et al. 2015; Morris and Maes 2013c; Maes et al. 2012b; Milrad et al. 2017; Montoya et al. 2017; Peterson et al. 2015; Russell et al. 2016). In addition, such different immune patterns may be explained by activation of the compensatory anti-inflammatory reflex system in patients with CFS, including Th-2 and Treg responses and induced immune tolerance (Morris and Maes 2012). This system tends to attenuate the primary immune response thus causing differential immune patterns depending on the stage of illness (e.g. acute episode versus chronic stage). Both concepts could also explain the results of large studies suggesting the development of a progressively hypo-inflammatory state in CFS patients with time and/or over the course of their disease both in terms of cytokine production and surface receptors on PMBCs (Hardcastle et al. 2015; Hornig et al. 2015; Montoya et al. 2017; Russell et al. 2016). Readers interested in further consideration of the different cytokine and PMBC receptor distribution patterns reported in CFS patterns are invited to consult reviews by (Morris and Maes 2013b; Morris et al. 2015a).
The existence of a state akin to endotoxin tolerance could also potentially explain a considerable body of data indicating the presence of exhausted or anergic CD4 and CD8 cells in CFS patients (Brenu et al. 2011, 2016; Klimas et al. 1990; Loebel et al. 2014; Prieto et al. 1989; Straus et al. 1993). This is also true of elevated Foxp3+ Tregs which appear to have been universally reported by researchers using advanced flow cytometry techniques (Brenu et al. 2011, 2014b; Curriu et al. 2013; Ramos et al. 2016). There have also been conflicting accounts of CD4 and CD8 T cell numbers both in absolute terms and in relation to each other which could conceivably be explained by patients being in different phases of an illness at the time of testing (Gupta and Vayuvegula 1991; Maes et al. 2015; Natelson et al. 2002; Racciatti et al. 2004). In this context, the work of Maes et al. and of Racciatti et al. would appear to be especially worthy of note as these studies were large, containing 139 and 134 patients respectively, and produced diametrically opposite results with the former reporting a reduced CD4/CD8 ratio while the latter reported an elevated CD4/CD8 ratio (Maes et al. 2015; Racciatti et al. 2004).
NK cell hypofunction is the most well documented immune abnormality in CFS patients (Brenu et al. 2016; Fletcher et al. 2010; Natelson et al. 2002) and there is evidence of abnormal metabolic pathways and impaired signalling activity known to be involved in endotoxin tolerance in this cell type extracted from CFS/ME patients both in terms of miRNA activity and impaired extracellular signal-regulated kinase 1/2, mitogen-activated protein kinase 1/2 and p38 signalling (Huth et al. 2016; Petty et al. 2016). It is also noteworthy that while there is a paucity of research examining macrophages, monocytes and neutrophils, those research teams which have investigated these parameters have reported abnormalities. For example, research teams have reported impaired phagocytosis and evidence of immune senescence in monocytes characterised by reduced expression of HLA-DR antigens (Gupta and Vayuvegula 1991; Prieto-Domínguez et al. 2016; Straus et al. 1993) while a recent study revealed a decrease in DCs overall but an increase in myeloid DCs, which is a characteristic of DC populations in a state of endotoxin tolerance as previously discussed (Brenu et al. 2014b). Several abnormalities in neutrophil phenotype and function have also been reported with reduced respiratory burst and phagocytic capacity together with an increased tendency to apoptosis being the most commonly reported findings (Brenu et al. 2010; Bryceson et al. 2006; Harvey et al. 2016; Kennedy et al. 2004). Other evidence of immune downregulation in at least some patients with CFS/ME includes downregulated TLR4 activity (Light et al. 2009, 2013; White et al. 2012) and a pattern of miRNA expression consistent with immune suppression induced by TGF-β1 signalling (Brenu et al. 2014a; Petty et al. 2016).
It is important to note that while large studies invariably report a plethora of immune abnormalities in patients with CFS/ME (Brenu et al. 2011; Hornig et al. 2015, 2017; Maes et al. 2012a, b, 2015; Milrad et al. 2017; Montoya et al. 2017; Peterson et al. 2015; Racciatti et al. 2004; Russell et al. 2016; Tirelli et al. 1993, 1994), this not true of smaller studies where the results are inconsistent even when participants fulfil the criteria for a CFS diagnosis according to international guidelines (reviewed (Natelson et al. 2002)). The reasons for such conflicting results are not entirely clear but the inclusion of individuals with primary depression, retrospective diagnoses and inconsistencies with cytokine assay approaches have all been cited as possible explanations. In addition, there is no evidence of any immune abnormalities in individuals afforded a diagnosis of CFS or ME based on any diagnostic schema other than the Fukuda or Canadian criteria (Blundell et al. 2015).
Conclusion
In this paper, it has been shown that, while the aetiology of CFS/ME is currently unknown, there is strong evidence of this illness being associated with a wide range of biological abnormalities, most notably in the neuroendocrine, autonomic, neurological, bioenergetic, redox and immunological domains. It has also been seen that epigenetic variation in immune response genes plays a major role in determining the development of DAMPs post-infection, which is pertinent from the perspective of the aetiology of the illness as the production or presence of these molecules can ‘convert’ an acute pathogenic infection into a state of escalating chronic systemic inflammation, which in turn can give rise to many of the reported symptoms and biological abnormalities. It has further been demonstrated in this paper how this relatively simple concept does indeed lead to a novel explanatory model which explains the major biological observations.
References
Abareshi A, Anaeigoudari A, Norouzi F, Shafei MN, Boskabady MH, Khazaei M, Hosseini M (2016) Lipopolysaccharide-induced spatial memory and synaptic plasticity impairment is preventable by captopril. Adv Med 2016:7676512. https://doi.org/10.1155/2016/7676512
Adam N, Kandelman S, Mantz J, Chretien F, Sharshar T (2013) Sepsis-induced brain dysfunction. Expert Rev Anti-Infect Ther 11:211–221. https://doi.org/10.1586/eri.12.159
Adriouch S, Hubert S, Pechberty S, Koch-Nolte F, Haag F, Seman M (2007) NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J Immunol 179:186–194. https://doi.org/10.4049/jimmunol.179.1.186
Ahn BH et al (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105:14447–14452. https://doi.org/10.1073/pnas.0803790105
Albrecht V, Hofer TP, Foxwell B, Frankenberger M, Ziegler-Heitbrock L (2008) Tolerance induced via TLR2 and TLR4 in human dendritic cells: role of IRAK-1. BMC Immunol 9:69. https://doi.org/10.1186/1471-2172-9-69
Alexeev EE, Lanis JM, Schwisow KD, Kominsky DJ, Colgan SP (2016) Microbiota-derived tryptophan metabolites activate aryl hydrocarbon receptor and induce IL-10 receptor expression in intestinal epithelia. FASEB J 30:57.52–57.52. https://doi.org/10.1096/fasebj.30.1_supplement.57.2
Aliev G et al (2010) Oxidative stress induced mitochondrial failure and vascular hypoperfusion as a key initiator for the development of Alzheimer disease. Pharmaceuticals (Basel, Switzerland) 3:158–187. https://doi.org/10.3390/ph3010158
Aliev G et al (2014) Oxidative stress mediated mitochondrial and vascular lesions as markers in the pathogenesis of Alzheimer disease. Curr Med Chem 21:2208–2217
Almeida A, Almeida J, Bolanos J, Moncada S (2001) Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci U S A 98:15294–15299
Al-Sadi R, Boivin M, Ma T (2009) Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci 14:2765–2778
Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ (2008) The role of neutrophils in severe sepsis. Shock (Augusta, Ga) 30(Suppl 1):3–9. https://doi.org/10.1097/SHK.0b013e3181818466
Anders HJ, Schaefer L (2014) Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25:1387–1400. https://doi.org/10.1681/asn.2014010117
Angrisano T et al (2010) LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol 10:172. https://doi.org/10.1186/1471-2180-10-172
Ansari A, Rahman MS, Saha SK, Saikot FK, Deep A, Kim KH (2017) Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 16:4–16. https://doi.org/10.1111/acel.12538
Antoniades C (2006) 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 114:1193–1201. https://doi.org/10.1161/circulationaha.106.612325
Araki K, Youngblood B, Ahmed R (2013) Programmed cell death 1-directed immunotherapy for enhancing T-cell function. Cold Spring Harb Symp Quant Biol 78:239–247. https://doi.org/10.1101/sqb.2013.78.019869
Ariga SK, Abatepaulo FB, Melo ES, Velasco IT, Pinheiro da Silva F, de Lima TM, Soriano FG (2014) Endotoxin tolerance drives neutrophil to infectious site. Shock 42:168–173. https://doi.org/10.1097/shk.0000000000000175
Armstrong CW, McGregor NR, Lewis DP, Butt HL, Gooley PR (2015) Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics 11:1626–1639. https://doi.org/10.1007/s11306-015-0816-5
Baas M et al (2016) TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. eLife 5:e08133. https://doi.org/10.7554/eLife.08133
Bai Y, Lu W, Han N, Bian H, Zhu M (2014) Functions of miR126 and innate immune response. Yi chuan = Hereditas 36:631–636. https://doi.org/10.3724/sp.j.1005.2014.0631
Banan A, Farhadi A, Fields JZ, Zhang LJ, Shaikh M, Keshavarzian A (2003) The delta-isoform of protein kinase C causes inducible nitric-oxide synthase and nitric oxide up-regulation: key mechanism for oxidant-induced carbonylation, nitration, and disassembly of the microtubule cytoskeleton and hyperpermeability of barrier of intestinal epithelia. J Pharmacol Exp Ther 305:482–494. https://doi.org/10.1124/jpet.102.047308
Banerjee R (2012) Redox outside the box: linking extracellular redox remodeling with intracellular redox metabolism. J Biol Chem 287:4397–4402. https://doi.org/10.1074/jbc.R111.287995
Banerjee S et al (2013) Morphine induced exacerbation of sepsis is mediated by tempering endotoxin tolerance through modulation of miR-146a. Sci Rep 3:1977. https://doi.org/10.1038/srep01977 https://www.nature.com/articles/srep01977#supplementary-information
Barnden L, Crouch B, Kwiatek R, Burnet R, Mernone A, Chryssidis S (2011) A brain MRI study of chronic fatigue syndrome: evidence of brainstem dysfunction and altered homeostasis. NMR Biomed 24:1302–1312
Barnden LR, Crouch B, Kwiatek R, Burnet R, Del Fante P (2015) Evidence in chronic fatigue syndrome for severity-dependent upregulation of prefrontal myelination that is independent of anxiety and depression. NMR Biomed 28:404–413. https://doi.org/10.1002/nbm.3261
Beaumont A, Burton AR, Lemon J, Bennett BK, Lloyd A, Vollmer-Conna U (2012) Reduced cardiac vagal modulation impacts on cognitive performance in chronic fatigue syndrome. PLoS One 7:e49518. https://doi.org/10.1371/journal.pone.0049518
Berk M et al (2013) So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 11:200. https://doi.org/10.1186/1741-7015-11-200
Bessede A et al (2014) Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511:184–190. https://doi.org/10.1038/nature13323
Biancardi VC, Stranahan AM, Krause EG, de Kloet AD, Stern JE (2016) Cross talk between AT1 receptors and toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am J Phys Heart Circ Phys 310:H404–H415. https://doi.org/10.1152/ajpheart.00247.2015
Billing-Ross P, Germain A, Ye K, Keinan A, Gu Z, Hanson MR (2016) Mitochondrial DNA variants correlate with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med 14:19. https://doi.org/10.1186/s12967-016-0771-6
Biswal B, Kunwar P, Natelson BH (2011) Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci 301:9–11. https://doi.org/10.1016/j.jns.2010.11.018
Biswas SK, Lopez-Collazo E (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 30:475–487. https://doi.org/10.1016/j.it.2009.07.009
Blundell S, Ray KK, Buckland M, White PD (2015) Chronic fatigue syndrome and circulating cytokines: a systematic review. Brain Behav Immun 50:186–195. https://doi.org/10.1016/j.bbi.2015.07.004
Bogdan C (2001) Nitric oxide and the regulation of gene expression. Trends Cell Biol 11:66–75
Boissoneault J, Letzen J, Lai S, O'Shea A, Craggs J, Robinson ME, Staud R (2016) Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: an arterial spin-labeling fMRI study. Magn Reson Imaging 34:603–608. https://doi.org/10.1016/j.mri.2015.12.008
Bolanos J, Almeida A, Moncada S (2010) Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci 35:145–149
Boneva RS et al (2007) Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: a population-based study. Auton Neurosci 137:94–101. https://doi.org/10.1016/j.autneu.2007.08.002
Boomer JS et al (2011) Immunosuppression in patients who die of sepsis and multiple organ failure. Jama 306:2594–2605. https://doi.org/10.1001/jama.2011.1829
Boomer JS, Green JM, Hotchkiss RS (2014) The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence 5:45–56. https://doi.org/10.4161/viru.26516
Borovikova LV et al (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462. https://doi.org/10.1038/35013070
Bouloumie A, Schini-Kerth VB, Busse R (1999) Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res 41:773–780
Bowie AG (2013) Removing the TREX1 safety net: oxidized DNA overcomes immune silencing by exonuclease TREX1. Immunity 39:423–425. https://doi.org/10.1016/j.immuni.2013.08.023
Bozzini (2012) The possible underworld of chronic fatigue syndrome from neurotransmitters polymorphisms to disease. J Neurol Res. https://doi.org/10.4021/jnr86w
Brenchley JM, Douek DC (2008) The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS 3:356–361. https://doi.org/10.1097/coh.0b013e3282f9ae9c
Brenu EW, Staines DR, Baskurt OK, Ashton KJ, Ramos SB, Christy RM, Marshall-Gradisnik SM (2010) Immune and hemorheological changes in chronic fatigue syndrome. J Transl Med 8:1. https://doi.org/10.1186/1479-5876-8-1
Brenu EW et al (2011) Immunological abnormalities as potential biomarkers in chronic fatigue syndrome/Myalgic encephalomyelitis. J Transl Med 9:81. https://doi.org/10.1186/1479-5876-9-81
Brenu EW, Ashton KJ, Batovska J, Staines DR, Marshall-Gradisnik SM (2014a) High-throughput sequencing of plasma microRNA in chronic fatigue syndrome/myalgic encephalomyelitis. PLoS One 9:e102783. https://doi.org/10.1371/journal.pone.0102783
Brenu EW et al (2014b) Role of adaptive and innate immune cells in chronic fatigue syndrome/myalgic encephalomyelitis. Int Immunol 26:233–242. https://doi.org/10.1093/intimm/dxt068
Brenu EW, Broadley S, Nguyen T, Johnston S, Ramos S, Staines D, Marshall-Gradisnik S (2016) A preliminary comparative assessment of the role of CD8+ T cells in chronic fatigue syndrome/Myalgic encephalomyelitis and multiple sclerosis. J Immunol Res 2016:8. https://doi.org/10.1155/2016/9064529
Brkic S, Tomic S, Maric D, Novakov Mikic A, Turkulov V (2010) Lipid peroxidation is elevated in female patients with chronic fatigue syndrome. Med Sci Monit 16:Cr628–Cr632
Bronkhorst MW, Boye ND, Lomax MA, Vossen RH, Bakker J, Patka P, Van Lieshout EM (2013) Single-nucleotide polymorphisms in the toll-like receptor pathway increase susceptibility to infections in severely injured trauma patients. J Trauma Acute Care Surg 74:862–870. https://doi.org/10.1097/TA.0b013e31827e1534
Brown AE, Jones DE, Walker M, Newton JL (2015) Abnormalities of AMPK activation and glucose uptake in cultured skeletal muscle cells from individuals with chronic fatigue syndrome. PLoS One 10:e0122982. https://doi.org/10.1371/journal.pone.0122982
Brunoni AR et al (2013) Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. Int J Neuropsychopharmacol 16:1937–1949. https://doi.org/10.1017/S1461145713000497
Brurberg KG, Fonhus MS, Larun L, Flottorp S, Malterud K (2014) Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): a systematic review. BMJ Open 4:e003973. https://doi.org/10.1136/bmjopen-2013-003973
Bryceson YT, March ME, Ljunggren HG, Long EO (2006) Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev 214:73–91. https://doi.org/10.1111/j.1600-065X.2006.00457.x
Burghardt KJ, Grove TB, Ellingrod VL (2013) Endothelial nitric oxide synthetase genetic variants, metabolic syndrome and endothelial function in schizophrenia. J Psychopharmacol 28:349–356. https://doi.org/10.1177/0269881113516200
Burton AR, Rahman K, Kadota Y, Lloyd A, Vollmer-Conna U (2010) Reduced heart rate variability predicts poor sleep quality in a case-control study of chronic fatigue syndrome. Exp Brain Res 204:71–78. https://doi.org/10.1007/s00221-010-2296-1
Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS (2006) Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394:627–634. https://doi.org/10.1042/bj20051435
Cabrera-Perez J, Condotta SA, Badovinac VP, Griffith TS (2014) Impact of sepsis on CD4 T cell immunity. J Leukoc Biol 96:767–777. https://doi.org/10.1189/jlb.5MR0114-067R
Calabrese M, Magliozzi R, Ciccarelli O, Geurts JJG, Reynolds R, Martin R (2015) Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci 16:147–158. https://doi.org/10.1038/nrn3900
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R (2008) Changes in gut microbiota control metabolic Endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481. https://doi.org/10.2337/db07-1403
Cani PD et al (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091–1103. https://doi.org/10.1136/gut.2008.165886
Cao C, Ma T, Y-f C, S-t S (2015) The role of regulatory T cells in immune dysfunction during sepsis. World J Emerg Med 6:5–9. https://doi.org/10.5847/wjem.j.1920-8642.2015.01.001
Carlo-Stella N et al (2006) A first study of cytokine genomic polymorphisms in CFS: positive association of TNF-857 and IFNgamma 874 rare alleles. Clin Exp Rheumatol 24:179–182
Carré JE, Singer M (2008) Cellular energetic metabolism in sepsis: the need for a systems approach. Biochim Biophy Acta 1777:763–771. https://doi.org/10.1016/j.bbabio.2008.04.024
Carruthers B et al (2011) Myalgic encephalomyelitis: international consensus criteria. J Intern Med 270:327–338
Carson WFT, Kunkel SL (2017) Regulation of cellular immune responses in sepsis by histone modifications. Adv Protein Chem Struct Biol 106:191–225. https://doi.org/10.1016/bs.apcsb.2016.08.004
Cha L, Jones AP, Trend S, Byrne SN, Fabis-Pedrini MJ, Carroll WM, Lucas RM, Cole JM, Booth DR, Kermode AG, Hart PH (2018) Tryptophan and arginine catabolic enzymes and regulatory cytokines in clinically isolated syndrome and multiple sclerosis. Clin Transl Immunol 7:e1037. https://doi.org/10.1002/cti2.1037
Chang J, Kunkel SL, Chang CH (2009) Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc Natl Acad Sci U S A 106:18327–18332. https://doi.org/10.1073/pnas.0905815106
Chaudhuri A, Condon B, Gow J, Brennan D, Hadley D (2003) Proton magnetic resonance spectroscopy of basal ganglia in chronic fatigue syndrome. Brain Imaging 14:225–228
Chen X, El Gazzar M, Yoza BK, McCall CE (2009) The NF-κB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance. J Biol Chem 284:27857–27865. https://doi.org/10.1074/jbc.M109.000950
Chen W et al (2010) Peroxynitrite Induces Destruction of the Tetrahydrobiopterin and Heme in Endothelial Nitric Oxide Synthase: Transition from Reversible to Irreversible Enzyme Inhibition. Biochemistry 49:3129–3137. https://doi.org/10.1021/bi9016632
Chen Z et al (2014) Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat Commun 5:4486. https://doi.org/10.1038/ncomms5486 https://www.nature.com/articles/ncomms5486#supplementary-information
Chen HL et al (2015) White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 38:361–370. https://doi.org/10.5665/sleep.4490
Cheng S-C et al (2014a) mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345. https://doi.org/10.1126/science.1250684
Cheng T, Hu C, Yang H, Cao L, An J (2014b) Transforming growth factor-beta-induced miR143 expression in regulation of non-small cell lung cancer cell viability and invasion capacity in vitro and in vivo. Int J Oncol 45:1977–1988. https://doi.org/10.3892/ijo.2014.2623
Cheng M-Y et al (2016) SIRT6 suppresses mitochondrial defects and cell death via the NF-κB pathway in myocardial hypoxia/reoxygenation induced injury. Am J Transl Res 8:5005–5015
Cheriyan J, Kim S, Wolansky LJ, Cook SD, Cadavid D (2012) Impact of inflammation on brain volume in multiple sclerosis. Arch Neurol 69:82–88. https://doi.org/10.1001/archneurol.2011.674
Chia JK, Chia AY (2008) Chronic fatigue syndrome is associated with chronic enterovirus infection of the stomach. J Clin Pathol 61. https://doi.org/10.1136/jcp.2007.050054
Chia J, Chia A, Voeller M, Lee T, Chang R (2010) Acute enterovirus infection followed by myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence. J Clin Pathol 63:165–168. https://doi.org/10.1136/jcp.2009.070466
Chiang P-L et al (2017) White matter damage and systemic inflammation in Parkinson’s disease. BMC Neurosci 18:48. https://doi.org/10.1186/s12868-017-0367-y
Chiche L et al (2011) The role of natural killer cells in sepsis. J Biomed Biotechnol 2011:986491. https://doi.org/10.1155/2011/986491
Ciregia F et al (2016) Bottom-up proteomics suggests an association between differential expression of mitochondrial proteins and chronic fatigue syndrome. Transl Psychiatry 6:e904. https://doi.org/10.1038/tp.2016.184
Clark LV et al (2017) Cytokine responses to exercise and activity in patients with chronic fatigue syndrome: case-control study. Clin Exp Immunol 190:360–371. https://doi.org/10.1111/cei.13023
Clements GB, McGarry F, Nairn C, Galbraith DN (1995) Detection of enterovirus-specific RNA in serum: the relationship to chronic fatigue. J Med Virol 45:156–161
Cockshell SJ, Mathias JL (2010) Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med 40:1253–1267. https://doi.org/10.1017/s0033291709992054
Cohen N, Morisset J, Emilie D (2004) Induction of tolerance by Porphyromonas gingivalis on APCS: a mechanism implicated in periodontal infection. J Dent Res 83:429–433. https://doi.org/10.1177/154405910408300515
Colasanti A et al (2016) Hippocampal Neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol Psychiatry 80:62–72. https://doi.org/10.1016/j.biopsych.2015.11.022
Cook DB et al (2017) Neural consequences of post-exertion malaise in Myalgic encephalomyelitis/chronic fatigue syndrome. Brain Behav Immun 62:87–99. https://doi.org/10.1016/j.bbi.2017.02.009
Cooper TM, McKinley PS, Seeman TE, Choo T-H, Lee S, Sloan RP (2015) Heart rate variability predicts levels of inflammatory markers: evidence for the vagal anti-inflammatory pathway. Brain Behav Immun 49:94–100. https://doi.org/10.1016/j.bbi.2014.12.017
Costa D, Tannock C, Brostoff J (1995) Brainstem perfusion is impaired in chronic fatigue syndrome. QJM 88:767–773
Cunningham L, Bowles NE, Archard LC (1991) Persistent virus infection of muscle in postviral fatigue syndrome. Br Med Bull 47:852–871
Curriu M et al (2013) Screening NK-, B- and T-cell phenotype and function in patients suffering from chronic fatigue syndrome. J Transl Med 11:68. https://doi.org/10.1186/1479-5876-11-68
Cvejic E, Lemon J, Hickie IB, Lloyd AR, Vollmer-Conna U (2014) Neurocognitive disturbances associated with acute infectious mononucleosis, Ross River fever and Q fever: a preliminary investigation of inflammatory and genetic correlates. Brain Behav Immun 36:207–214. https://doi.org/10.1016/j.bbi.2013.11.002
Cvejic E, Birch RC, Vollmer-Conna U (2016) Cognitive dysfunction in chronic fatigue syndrome: a review of recent evidence. Curr Rheumatol Rep 18:24. https://doi.org/10.1007/s11926-016-0577-9
Danahy DB, Strother RK, Badovinac VP, Griffith TS (2016) Clinical and experimental Sepsis impairs CD8 T-cell-mediated immunity. Crit Rev Immunol 36:57–74. https://doi.org/10.1615/CritRevImmunol.2016017098
de Castilho FM, Ribeiro ALP, da Silva JLP, Nobre V, de Sousa MR (2017) Heart rate variability as predictor of mortality in sepsis: a prospective cohort study. PLoS One 12:e0180060. https://doi.org/10.1371/journal.pone.0180060
de Lange F, Kalkman J, Bleijenberg G, Hagoort P, van der Meer J, Toni I (2005) Gray matter volume reduction in the chronic fatigue syndrome. Neuroimage 26:777–781
de Lange F, Koers A, Kalkman J, Bleijenberg G, Hagoort P, van der Meer J (2008) Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain 131:2172–2180
de Souza AR, Zago M, Eidelman DH, Hamid Q, Baglole CJ (2014) Aryl hydrocarbon receptor (AhR) attenuation of subchronic cigarette smoke-induced pulmonary neutrophilia is associated with retention of nuclear RelB and suppression of intercellular adhesion Molecule-1 (ICAM-1). Toxicol Sci 140:204–223. https://doi.org/10.1093/toxsci/kfu068
de Vega WC, Vernon SD, McGowan PO (2014) DNA methylation modifications associated with chronic fatigue syndrome. PLoS One 9:e104757. https://doi.org/10.1371/journal.pone.0104757
de Vega WC, Herrera S, Vernon SD, McGowan PO (2017) Epigenetic modifications and glucocorticoid sensitivity in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). BMC Med Genet 10:11. https://doi.org/10.1186/s12920-017-0248-3
del Campo R et al (2011) Translocated LPS might cause endotoxin tolerance in circulating monocytes of cystic fibrosis patients. PLoS One 6:e29577. https://doi.org/10.1371/journal.pone.0029577
del Fresno C, Gomez-Garcia L, Caveda L, Escoll P, Arnalich F, Zamora R, Lopez-Collazo E (2004) Nitric oxide activates the expression of IRAK-M via the release of TNF-alpha in human monocytes. Nitric Oxide 10:213–220. https://doi.org/10.1016/j.niox.2004.04.007
del Fresno C et al (2007) Inflammatory responses associated with acute coronary syndrome up-regulate IRAK-M and induce endotoxin tolerance in circulating monocytes. J Endotoxin Res 13:39–52. https://doi.org/10.1177/0968051907078623
del Fresno C et al (2008) Monocytes from cystic fibrosis patients are locked in an LPS tolerance state: down-regulation of TREM-1 as putative underlying mechanism. PLoS One 3:e2667. https://doi.org/10.1371/journal.pone.0002667
del Fresno C et al (2009) Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol (Baltimore, Md : 1950) 182:6494–6507. https://doi.org/10.4049/jimmunol.0803350
DelaTorre A, Schroeder RA, Kuo PC (1997) Alteration of NF-κB p50 DNA binding kinetics by S-Nitrosylation. Biochem Biophys Res Commun 238:703–706. https://doi.org/10.1006/bbrc.1997.7279
DelaTorre A, Schroeder RA, Bartlett ST, Kuo PC (1998) Differential effects of nitric oxide-mediated S-nitrosylation on p50 and c-jun DNA binding. Surgery 124:137–141
DelaTorre A, Schroeder RA, Punzalan C, Kuo PC (1999) Endotoxin-mediated S-nitrosylation of p50 alters NF-kappa B-dependent gene transcription in ANA-1 murine macrophages. J Immunol 162:4101–4108
DeLuca J, Christodoulou C, Diamond BJ, Rosenstein ED, Kramer N, Natelson BH (2004) Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc 10:101-109.https://doi.org/10.1017/s1355617704101124
Delzenne NM, Cani PD (2011) Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr 31:15–31. https://doi.org/10.1146/annurev-nutr-072610-145146
Dimmock ME, Mirin AA, Jason LA (2016) Estimating the disease burden of ME/CFS in the United States and its relation to research funding. J Med Ther 1:1–7. https://doi.org/10.15761/JMT.1000102
Domogalla MP, Rostan PV, Raker VK, Steinbrink K (2017) Tolerance through education: how Tolerogenic dendritic cells shape immunity. Front Immunol 8:1764. https://doi.org/10.3389/fimmu.2017.01764
Doulias PT, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H (2013) Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci Signal 6(256):rs1. https://doi.org/10.1126/scisignal.2003252
Drexler SK, Foxwell BM (2010) The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol 42:506–518. https://doi.org/10.1016/j.biocel.2009.10.009
Drose S, Brandt U, Wittig I (2014) Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim Biophys Acta 1844:1344–1354. https://doi.org/10.1016/j.bbapap.2014.02.006
Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, Badovinac VP (2014) Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J Immunol (Baltimore, Md : 1950) 192:3618–3625. https://doi.org/10.4049/jimmunol.1303460
Durosier LD et al (2015) Does heart rate variability reflect the systemic inflammatory response in a fetal sheep model of lipopolysaccharide-induced sepsis? Physiol Meas 36:2089–2102. https://doi.org/10.1088/0967-3334/36/10/2089
Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17:162–184. https://doi.org/10.1016/j.cmet.2012.12.012
Elfaitouri A et al (2013) Epitopes of microbial and human heat shock protein 60 and their recognition in Myalgic encephalomyelitis. PLoS One 8:e81155. https://doi.org/10.1371/journal.pone.0081155
Elhanati S et al (2016) Reciprocal regulation between SIRT6 and miR-122 controls liver metabolism and predicts Hepatocarcinoma prognosis. Cell Rep 14:234–242. https://doi.org/10.1016/j.celrep.2015.12.023
Escoll P, del Fresno C, Garcia L, Valles G, Lendinez MJ, Arnalich F, Lopez-Collazo E (2003) Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem Biophys Res Commun 311:465–472
Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH (2015) Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. https://doi.org/10.1038/mp.2015.168
Ferretti C, La Cava A (2014) miR-126, a new modulator of innate immunity. Cell Mol Immunol 11:215–217. https://doi.org/10.1038/cmi.2014.5
Fichna M, Żurawek M, Fichna P, Januszkiewicz-Lewandowska D, Ruchała M, Nowak J (2016) Polymorphisms of the toll-like Receptor-3 gene in autoimmune adrenal failure and type 1 diabetes in polish patients. Arch Immunol Ther Exp 64:83–87. https://doi.org/10.1007/s00005-015-0360-z
Finkelmeyer A, He J, Maclachlan L, Watson S, Gallagher P, Newton JL, Blamire AM (2018) Grey and white matter differences in chronic fatigue syndrome – a voxel-based morphometry study. NeuroImage Clin 17:24–30. https://doi.org/10.1016/j.nicl.2017.09.024
Fischler B et al (1996) Comparison of 99m Tc HMPAO SPECT scan between chronic fatigue syndrome, major depression and healthy controls: an exploratory study of clinical correlates of regional cerebral blood flow. Neuropsychobiol 34:175–183
Fletcher MA et al (2010) Biomarkers in chronic fatigue syndrome: evaluation of natural killer cell function and dipeptidyl peptidase IV/CD26. PLoS One 5. https://doi.org/10.1371/journal.pone.0010817
Fluge Ø et al (2016) Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight 1:e89376. https://doi.org/10.1172/jci.insight.89376
Forel JM et al (2012) Phenotype and functions of natural killer cells in critically-ill septic patients. PLoS One 7:e50446. https://doi.org/10.1371/journal.pone.0050446
Foster SL, Hargreaves DC, Medzhitov R (2007) Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447:972. https://doi.org/10.1038/nature05836 https://www.nature.com/articles/nature05836#supplementary-information
Frost RA, Lang CH (2011) mTOR signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda, Md) 26:83–96. https://doi.org/10.1152/physiol.00044.2010
Fu Y et al (2012) Network topologies and dynamics leading to endotoxin tolerance and priming in innate immune cells. PLoS Comput Biol 8:e1002526. https://doi.org/10.1371/journal.pcbi.1002526
Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A (1994) The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 121:953-959.https://doi.org/10.7326/0003-4819-121-12-199412150-00009
Fulle S et al (2000) Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome. Free Radic Biol Med 29:1252–1259
Fulle S, Pietrangelo T, Mancinelli R, Saggini R, Fano G (2007) Specific correlations between muscle oxidative stress and chronic fatigue syndrome: a working hypothesis. J Muscle Res Cell Motil 28:355–362
Gandhi R et al (2010) Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell–like and Foxp3+ regulatory T cells. Nat Immunol 11:846. https://doi.org/10.1038/ni.1915 https://www.nature.com/articles/ni.1915#supplementary-information
Gay CW, Robinson ME, Lai S, O'Shea A, Craggs JG, Price DD, Staud R (2016) Abnormal resting-state functional connectivity in patients with chronic fatigue syndrome: results of seed and data-driven analyses. Brain Connect 6:48–56. https://doi.org/10.1089/brain.2015.0366
Gazzar ME, Yoza BK, Chen X, Hu J, Hawkins GA, McCall CE (2008) G9a and HP1 couple histone and DNA methylation to TNFα transcription silencing during endotoxin tolerance. J Biol Chem 283:32198–32208. https://doi.org/10.1074/jbc.M803446200
Gazzar ME, Yoza BK, Chen X, Garcia BA, Young NL, McCall CE (2009) Chromatin-specific remodeling by HMGB1 and linker histone H1 silences Proinflammatory genes during endotoxin tolerance. Mol Cell Biol 29:1959–1971. https://doi.org/10.1128/mcb.01862-08
Germain A, Ruppert D, Levine SM, Hanson MR (2017) Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol BioSyst 13:371–379. https://doi.org/10.1039/c6mb00600k
Gerwyn M, Maes M (2017) Mechanisms explaining muscle fatigue and muscle pain in patients with Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a review of recent findings. Curr Rheumatol Rep 19:1. https://doi.org/10.1007/s11926-017-0628-x
Ghosh HS, McBurney M, Robbins PD (2010) SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5:e9199. https://doi.org/10.1371/journal.pone.0009199
Gil R, Barth S, Kanfi Y, Cohen HY (2013) SIRT6 exhibits nucleosome-dependent deacetylase activity. Nucleic Acids Res 41:8537–8545. https://doi.org/10.1093/nar/gkt642
Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR (2000) Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci 85:49–59. https://doi.org/10.1016/s1566-0702(00)00219-8
Goh FG, Midwood KS (2012) Intrinsic danger: activation of toll-like receptors in rheumatoid arthritis. Rheumatology (Oxford) 51:7–23. https://doi.org/10.1093/rheumatology/ker257
Goldstein JA, Mena I, Jouanne E, Lesser I (1995) The assessment of vascular abnormalities in late life chronic fatigue syndrome by brain SPECT. J Chronic Fatigue Syndr 1:55–79. https://doi.org/10.1300/J092v01n01_05
Gómez H, Jin K, Kellum JA (2015) The role of energy regulation in the tubular epithelial cell response to sepsis. Nephron 131:255–258. https://doi.org/10.1159/000437278
Gómez H, Kellum JA, Ronco C (2017) Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol 13:143–151. https://doi.org/10.1038/nrneph.2016.186
Gonzalez-Leon MC et al (2006) Nitric oxide induces SOCS-1 expression in human monocytes in a TNF-alpha-dependent manner. J Endotoxin Res 12:296–306. https://doi.org/10.1179/096805106x118843
Gorelenkova Miller O, Mieyal J (2015) Sulfhydryl-mediated redox signaling in inflammation: role in neurodegenerative diseases. Arch Toxicol 89:1439–1467. https://doi.org/10.1007/s00204-015-1496-7
Gorelick PB (2010) Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci 1207:155–162. https://doi.org/10.1111/j.1749-6632.2010.05726.x
Gow JW, Behan WM (1991) Amplification and identification of enteroviral sequences in the postviral fatigue syndrome. Br Med Bull 47:872–885
Gow JW, Hagan S, Herzyk P, Cannon C, Behan PO, Chaudhuri A (2009) A gene signature for post-infectious chronic fatigue syndrome. BMC Med Genet 2:38. https://doi.org/10.1186/1755-8794-2-38
Grigoryev YA et al (2011) MicroRNA regulation of molecular networks mapped by global MicroRNA, mRNA, and protein expression in activated T lymphocytes. J Immunol. https://doi.org/10.4049/jimmunol.1101233
Guo C, Sah FJ, Beard L, Willson JKV, Markowitz SD, Guda K (2008) The non-coding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in Colon cancers. Genes Chromosom Cancer 47:939–946. https://doi.org/10.1002/gcc.20596
Guo S, Al-Sadi R, Said HM, Ma TY (2013) Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol 182:375–387. https://doi.org/10.1016/j.ajpath.2012.10.014
Guo L, Zhang Y, Zhang L, Huang F, Li J, Wang S (2016) MicroRNAs, TGF-β signaling, and the inflammatory microenvironment in cancer. Tumour Biol 37:115–125. https://doi.org/10.1007/s13277-015-4374-2
Gupta S, Vayuvegula B (1991) A comprehensive immunological analysis in chronic fatigue syndrome. Scand J Immunol 33:319–327
Gupta A et al (2017) PARK2 depletion connects energy and oxidative stress to PI3K/Akt activation via PTEN S-Nitrosylation. Mol Cell 65:999–1013.e1017. https://doi.org/10.1016/j.molcel.2017.02.019
Haag F et al (2007) Extracellular NAD and ATP: partners in immune cell modulation. Purinergic Signal 3:71–81. https://doi.org/10.1007/s11302-006-9038-7
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Hanson MR, Gu Z, Keinan A, Ye K, Germain A, Billing-Ross P (2016) Association of mitochondrial DNA variants with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) symptoms. J Transl Med 14:342. https://doi.org/10.1186/s12967-016-1104-5
Hardcastle SL et al (2015) Longitudinal analysis of immune abnormalities in varying severities of chronic fatigue syndrome/Myalgic encephalomyelitis patients. J Transl Med 13:299. https://doi.org/10.1186/s12967-015-0653-3
Harvey JM et al (2016) Tracking post-infectious fatigue in clinic using routine lab tests. BMC Pediatr 16:54. https://doi.org/10.1186/s12887-016-0596-8
Helbig K, Harris R, Ayres J, Dunckley H, Lloyd A, Robson J, Marmion BP (2005) Immune response genes in the post-Q-fever fatigue syndrome, Q fever endocarditis and uncomplicated acute primary. Q Fever Qjm 98:565–574. https://doi.org/10.1093/qjmed/hci086
Hendrickson SL et al (2008) Mitochondrial DNA Haplogroups influence AIDS progression. AIDS (London, England) 22:2429–2439. https://doi.org/10.1097/QAD.0b013e32831940bb
Herlitz GN et al (2015) Physiologic variability at the verge of systemic inflammation: multi-scale entropy of heart rate variability is affected by very low doses of endotoxin. Shock (Augusta, Ga) 43:133–139. https://doi.org/10.1097/SHK.0000000000000276
Hermida MA, Dinesh Kumar J, Leslie NR (2017) GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul 65:5–15. https://doi.org/10.1016/j.jbior.2017.06.003
Herry CL et al (2016) Temporal patterns in sheep fetal heart rate variability correlate to systemic cytokine inflammatory response: a methodological exploration of monitoring potential using complex signals bioinformatics. PLoS One 11:e0153515. https://doi.org/10.1371/journal.pone.0153515
Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6:150–166. https://doi.org/10.1038/nrm1569
Hickie I et al (2006) Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 333. https://doi.org/10.1136/bmj.38933.585764.AE
Hill BG, Bhatnagar A (2012) Protein S-glutathiolation: redox-sensitive regulation of protein function. J Mol Cell Cardiol 52:559–567. https://doi.org/10.1016/j.yjmcc.2011.07.009
Hines DJ, Choi HB, Hines RM, Phillips AG, MacVicar BA (2013) Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PLoS One 8:e60388. https://doi.org/10.1371/journal.pone.0060388
Hokama Y et al (2008) Acute phase phospholipids related to the cardiolipin of mitochondria in the sera of patients with chronic fatigue syndrome (CFS), chronic ciguatera fish poisoning (CCFP), and other diseases attributed to chemicals, gulf war, and marine toxins. J Clin Lab Anal 22:99–105. https://doi.org/10.1002/jcla.20217
Hokama Y, Camproa C, Hara C, Kuribayashi T, Le Huynh D, Yabusaki K (2009) Anticardiolipin antibodies in the sera of patients with diagnosed chronic fatigue syndrome. J Clin Lab Anal 23:210–212
Hornig M et al (2015) Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv 1. https://doi.org/10.1126/sciadv.1400121
Hornig M, Gottschalk CG, Eddy ML, Che X, Ukaigwe JE, Peterson DL, Lipkin WI (2017) Immune network analysis of cerebrospinal fluid in myalgic encephalomyelitis/chronic fatigue syndrome with atypical and classical presentations. Transl Psychiatry 7:e1080. https://doi.org/10.1038/tp.2017.44 https://www.nature.com/articles/tp201744#supplementary-information
Huan L et al (2016) MicroRNA-127-5p targets the biliverdin reductase B/nuclear factor-κB pathway to suppress cell growth in hepatocellular carcinoma cells. Cancer Sci 107:258–266. https://doi.org/10.1111/cas.12869
Hughes AM, Hirsch CR, Nikolaus S, Chalder T, Knoop H, Moss-Morris R (2018) Cross-cultural study of information processing biases in chronic fatigue syndrome: comparison of Dutch and UK chronic fatigue patients. Int J Behav Med 25:49–54. https://doi.org/10.1007/s12529-017-9682-z
Huston JM, Tracey KJ (2011) The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway, and implications for therapy. J Intern Med 269:45–53. https://doi.org/10.1111/j.1365-2796.2010.02321.x
Huth TK, Staines D, Marshall-Gradisnik S (2016) ERK1/2, MEK1/2 and p38 downstream signalling molecules impaired in CD56 dim CD16+ and CD56 bright CD16 dim/− natural killer cells in chronic fatigue syndrome/Myalgic encephalomyelitis patients. J Transl Med 14:97. https://doi.org/10.1186/s12967-016-0859-z
Ichise M, Salit I, Abbey S, Chung D, Gray B, Kirsh J (1992) Assessment of regional cerebral perfusion by 99Tcm-HMPAO SPECT in chronic fatigue syndrome. Nucl Med Commun 13:767–772
Im E, Riegler FM, Pothoulakis C, Rhee SH (2012) Elevated lipopolysaccharide in the colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice. Am J Physiol Gastrointest Liver Physiol 303:G490–G497. https://doi.org/10.1152/ajpgi.00120.2012
Imanishi H et al (2013) Specific mtDNA mutations in mouse carcinoma cells suppress their tumor formation via activation of the host innate immune system. PLoS One 8:e75981. https://doi.org/10.1371/journal.pone.0075981
Into T, Inomata M, Nakashima M, Shibata K, Hacker H, Matsushita K (2008) Regulation of MyD88-dependent signaling events by S nitrosylation retards toll-like receptor signal transduction and initiation of acute-phase immune responses. Mol Cell Biol 28:1338–1347. https://doi.org/10.1128/mcb.01412-07
Ishikawa K et al (2010) The innate immune system in host mice targets cells with allogenic mitochondrial DNA. J Exp Med 207:2297–2305. https://doi.org/10.1084/jem.20092296
Ishiyama K, Ohdan H, Tokita D, Shishida M, Tanaka Y, Irei T, Asahara T (2006) Induction of endotoxin tolerance inhibits alloimmune responses. Transpl Immunol 16:158–165. https://doi.org/10.1016/j.trim.2006.06.002
Jammes Y, Steinberg J, Mambrini O, Bregeon F, Delliaux S (2005) Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med 257:299–310
Jammes Y, Steinberg JG, Delliaux S, Bregeon F (2009) Chronic fatigue syndrome combines increased exercise-induced oxidative stress and reduced cytokine and Hsp responses. J Intern Med 266:196–206. https://doi.org/10.1111/j.1365-2796.2009.02079.x
Jammes Y, Steinberg J, Delliaux S (2011) Chronic fatigue syndrome: acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins. J Intern Med 272:74–84
Jammes Y, Steinberg JG, Delliaux S (2012) Chronic fatigue syndrome: acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins. J Intern Med 272:74–84. https://doi.org/10.1111/j.1365-2796.2011.02488.x
Jan BU, Coyle SM, Macor MA, Reddell M, Calvano SE, Lowry SF (2010) Relationship of basal heart rate variability to in vivo cytokine responses after endotoxin exposure. Shock (Augusta, Ga) 33:363–368. https://doi.org/10.1097/SHK.0b013e3181b66bf4
Jang IJ et al (2017) Acute inflammation reveals GABA(A) receptor-mediated nociception in mouse dorsal root ganglion neurons via PGE (2) receptor 4 signaling. Phys Rep 5:e13178. https://doi.org/10.14814/phy2.13178
Jason LA, Brown A, Clyne E, Bartgis L, Evans M, Brown M (2012) Contrasting case definitions for chronic fatigue syndrome, myalgic encephalomyelitis/chronic fatigue syndrome and myalgic encephalomyelitis. Eval Health Prof 35:280–304. https://doi.org/10.1177/0163278711424281
Jason LA et al (2015a) Chronic fatigue syndrome and Myalgic encephalomyelitis: toward An empirical case definition. Health Psychol Behav Med 3:82–93. https://doi.org/10.1080/21642850.2015.1014489
Jason LA, Sunnquist M, Brown A, Reed J (2015b) Defining essential features of Myalgic encephalomyelitis and chronic fatigue syndrome. J Hum Behav Soc Environ 25:657–674. https://doi.org/10.1080/10911359.2015.1011256
Jason LA, McManimen S, Sunnquist M, Brown A, Furst J, Newton JL, Strand EB (2016) Case definitions integrating empiric and consensus perspectives. Fatigue 4:1–23. https://doi.org/10.1080/21641846.2015.1124520
Jeon SY et al (2017) [(11)C]-(R)-PK11195 positron emission tomography in patients with complex regional pain syndrome: a pilot study. Medicine 96:e5735. https://doi.org/10.1097/MD.0000000000005735
Jiang H, Chess L (2006) Regulation of immune responses by T cells. N Engl J Med 354:1166–1176. https://doi.org/10.1056/NEJMra055446
Jiang L-N, Yao Y-M, Sheng Z-Y (2012) The role of regulatory T cells in the pathogenesis of Sepsis and its clinical implication. J Interf Cytokine Res 32:341–349. https://doi.org/10.1089/jir.2011.0080
Jiang S et al (2014) Human resistin promotes neutrophil proinflammatory activation and neutrophil extracellular trap formation and increases severity of acute lung injury. J Immunol (Baltimore, Md : 1950) 192:4795–4803. https://doi.org/10.4049/jimmunol.1302764
Johnston GR, Webster NR (2009) Cytokines and the immunomodulatory function of the vagus nerve. Br J Anaesth. https://doi.org/10.1093/bja/aep037
Johnston S, Brenu EW, Staines D, Marshall-Gradisnik S (2013) The prevalence of chronic fatigue syndrome/ myalgic encephalomyelitis: a meta-analysis. Clin Epidemiol 5:105–110. https://doi.org/10.2147/CLEP.S39876
Kadota Y, Cooper G, Burton AR, Lemon J, Schall U, Lloyd A, Vollmer-Conna U (2010) Autonomic hyper-vigilance in post-infective fatigue syndrome. Biol Psychol 85:97–103. https://doi.org/10.1016/j.biopsycho.2010.05.009
Kakihana Y, Ito T, Nakahara M, Yamaguchi K, Yasuda T (2016) Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care 4:22. https://doi.org/10.1186/s40560-016-0148-1
Kalous KS, Wynia-Smith SL, Olp MD, Smith BC (2016) Mechanism of Sirt1 NAD+−dependent deacetylase inhibition by cysteine S-nitrosation. J Biol Chem. https://doi.org/10.1074/jbc.M116.754655
Kanjwal K, Karabin B, Kanjwal Y, Saeed B, Grubb BP (2010) Autonomic dysfunction presenting as orthostatic intolerance in patients suffering from mitochondrial cytopathy. Clin Cardiol 33:626–629. https://doi.org/10.1002/clc.20805
Karaa A, Rahman S, Lombès A, Yu-Wai-Man P, Sheikh MK, Alai-Hansen S, Cohen BH, Dimmock D, Emrick L, Falk MJ, McCormack S, Mirsky D, Moore T, Parikh S, Shoffner J, Taivassalo T, Tarnopolsky M, Tein I, Odenkirchen JC, Goldstein A, Mito Working Group Member Participants (2017) Common data elements for clinical research in mitochondrial disease: a National Institute for neurological disorders and stroke project. J Inherit Metab Dis 40:403–414. https://doi.org/10.1007/s10545-017-0035-5
Karaa A, Rahman S, Lombès A, Yu-Wai-Man P, Sheikh MK, Alai-Hansen S, Cohen BH, Dimmock D, Emrick L, Falk MJ, McCormack S, Mirsky D, Moore T, Parikh S, Shoffner J, Taivassalo T, Tarnopolsky M, Tein I, Odenkirchen JC, Goldstein A, Mito Working Group Member Participants (2018) Erratum to: common data elements for clinical research in mitochondrial disease: a National Institute for neurological disorders and stroke project. J Inherit Metab Dis 41:151. https://doi.org/10.1007/s10545-017-0081-z
Kasuno K et al (2004) Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem 279:2550–2558. https://doi.org/10.1074/jbc.M308197200
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A (2013) Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25:1939–1948. https://doi.org/10.1016/j.cellsig.2013.06.007
Kawahara TL et al (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136:62–74. https://doi.org/10.1016/j.cell.2008.10.052
Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE (2007) NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem 282:30667–30672. https://doi.org/10.1074/jbc.M705929200
Kennedy G, Spence V, Underwood C, Belch JJ (2004) Increased neutrophil apoptosis in chronic fatigue syndrome. J Clin Pathol 57:891–893. https://doi.org/10.1136/jcp.2003.015511
Kerr JR et al (2008a) Seven genomic subtypes of chronic fatigue syndrome/myalgic encephalomyelitis: a detailed analysis of gene networks and clinical phenotypes. J Clin Pathol 61:730–739. https://doi.org/10.1136/jcp.2007.053553
Kerr JR et al (2008b) Gene expression subtypes in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J Infect Dis 197. https://doi.org/10.1086/533453
Kim JW, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3:177–185. https://doi.org/10.1016/j.cmet.2006.02.002
Kim B-K, Yoo H-I, Choi K, Yoon SK (2015a) miR-330-5p inhibits proliferation and migration of keratinocytes by targeting Pdia3 expression. FEBS J 282:4692–4702. https://doi.org/10.1111/febs.13523
Kim BH, Namkoong K, Kim JJ, Lee S, Yoon KJ, Choi M, Jung YC (2015b) Altered resting-state functional connectivity in women with chronic fatigue syndrome. Psychiatry Res 234:292–297. https://doi.org/10.1016/j.pscychresns.2015.10.014
Kim S, Miller BJ, Stefanek ME, Miller AH (2015c) Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: relevance to cancer-related fatigue. Cancer 121:2129–2136. https://doi.org/10.1002/cncr.29302
Kincaid B, Bossy-Wetzel E (2013) Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci 5:48. https://doi.org/10.3389/fnagi.2013.00048
Klimas NG, Salvato FR, Morgan R, Fletcher MA (1990) Immunologic abnormalities in chronic fatigue syndrome. J Clin Microbiol 28:1403–1410
Kohr MJ, Evangelista AM, Ferlito M, Steenbergen C, Murphy E (2014) S-nitrosylation of TRIM72 at cysteine 144 is critical for protection against oxidation-induced protein degradation and cell death. J Mol Cell Cardiol 69:67–74. https://doi.org/10.1016/j.yjmcc.2014.01.010
Konsman JP, Parnet P, Dantzer R (2002) Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci 25:154–159
Kwak YD et al (2010) NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol Neurodegener 5:49. https://doi.org/10.1186/1750-1326-5-49
Labrenz F, Wrede K, Forsting M, Engler H, Schedlowski M, Elsenbruch S, Benson S (2016) Alterations in functional connectivity of resting state networks during experimental endotoxemia - An exploratory study in healthy men. Brain Behav Immun 54:17–26. https://doi.org/10.1016/j.bbi.2015.11.010
Laing KJ, Dong L, Sidney J, Sette A, Koelle DM (2012) Immunology in the clinic review series; focus on host responses: T cell responses to herpes simplex viruses. Clin Exp Immunol 167:47–58. https://doi.org/10.1111/j.1365-2249.2011.04502.x
Lane R, Soteriou B, Zhang H, Archard L (2003) Enterovirus related metabolic myopathy: a postviral fatigue syndrome. J Neurol Neurosurg Psychiatry 74:1382–1386
Lange G et al (2005) Objective evidence of cognitive complaints in chronic fatigue syndrome: a BOLD fMRI study of verbal working memory. NeuroImage 26:513–524. https://doi.org/10.1016/j.neuroimage.2005.02.011
Lanis JM et al (2017) Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol 10:1133. https://doi.org/10.1038/mi.2016.133 https://www.nature.com/articles/mi2016133#supplementary-information
Lauw FN, ten Hove T, Dekkers PE, de Jonge E, van Deventer SJ, van Der Poll T (2000) Reduced Th1, but not Th2, cytokine production by lymphocytes after in vivo exposure of healthy subjects to endotoxin. Infect Immun 68:1014–1018
Lavoie KL, Pelletier R, Arsenault A, Dupuis J, Bacon SL (2010) Association between clinical depression and endothelial function measured by forearm hyperemic reactivity. Psychosom Med 72:20–26. https://doi.org/10.1097/psy.0b013e3181c2d6b8
Lawson N, Hsieh CH, March D, Wang X (2016) Elevated Energy Production in Chronic Fatigue Syndrome Patients. J Nat Sci 2
Lee SH (2015) Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 13:11–18. https://doi.org/10.5217/ir.2015.13.1.11
Lee M, Choy JC (2013) Positive feedback regulation of human inducible nitric oxide synthase expression by Ras S-nitrosylation. J Biol Chem. https://doi.org/10.1074/jbc.M113.475319
Lee J, Ahn E, Kissick HT, Ahmed R (2015) Reinvigorating exhausted T cells by blockade of the PD-1 pathway. For Immunopath Dis Therap 6:7–17. https://doi.org/10.1615/ForumImmunDisTher.2015014188
Lehrer P et al (2010) Voluntarily produced increases in heart rate variability modulate autonomic effects of endotoxin induced systemic inflammation: an exploratory study. Appl Psychophysiol Biofeedback 35:303–315. https://doi.org/10.1007/s10484-010-9139-5
Leibundgut G, Witztum JL, Tsimikas S (2013) Oxidation-specific epitopes and immunological responses: translational biotheranostic implications for atherosclerosis. Curr Opin Pharmacol 13:168–179. https://doi.org/10.1016/j.coph.2013.02.005
Lekander M et al (2016) Intrinsic functional connectivity of insular cortex and symptoms of sickness during acute experimental inflammation. Brain Behav Immun 56:34–41. https://doi.org/10.1016/j.bbi.2015.12.018
Levy RJ, Piel DA, Acton PD, Zhou R, Ferrari VA, Karp JS, Deutschman CS (2005) Evidence of myocardial hibernation in the septic heart. Crit Care Med 33:2752–2756
Lewis I, Pairman J, Spickett G, Newton JL (2013) Clinical characteristics of a novel subgroup of chronic fatigue syndrome patients with postural orthostatic tachycardia syndrome. J Intern Med 273:501–510. https://doi.org/10.1111/joim.12022
Li F et al (2007) Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell 26:63–74. https://doi.org/10.1016/j.molcel.2007.02.024
Li H et al (2016a) Acupuncture reversed hippocampal mitochondrial dysfunction in vascular dementia rats. Neurochem Int 92:35–42. https://doi.org/10.1016/j.neuint.2015.12.001
Li Q, Harden JL, Anderson CD, Egilmez NK (2016b) Tolerogenic phenotype of IFN-gamma-induced IDO+ dendritic cells is maintained via an autocrine IDO-kynurenine/AhR-IDO loop. J Immunol (Baltimore, Md : 1950) 197:962–970. https://doi.org/10.4049/jimmunol.1502615
Li M et al (2017) Lentivirus-mediated interleukin-1beta (IL-1beta) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J Neuroinflammation 14:190. https://doi.org/10.1186/s12974-017-0964-9
Light A, White A, Hughen R, Light K (2009) Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain 10:1099–1112
Light AR, Bateman L, Jo D, Hughen RW, Vanhaitsma TA, White AT, Light KC (2012) Gene expression alterations at baseline and following moderate exercise in patients with chronic fatigue syndrome and fibromyalgia syndrome. J Intern Med 271:64–81. https://doi.org/10.1111/j.1365-2796.2011.02405.x
Light KC et al (2013) Differing leukocyte gene expression profiles associated with fatigue in patients with prostate cancer versus chronic fatigue syndrome. Psychoneuroendocrinology 38:2983–2995. https://doi.org/10.1016/j.psyneuen.2013.08.008
Limaye AP et al (2008) Cytomegalovirus reactivation in critically ill immunocompetent patients. Jama 300:413–422. https://doi.org/10.1001/jama.300.4.413
Littman DR, Rudensky AY (2010) Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140:845–858. https://doi.org/10.1016/j.cell.2010.02.021
Litzenburger UM et al (2014) Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 5:1038–1051
Liu H, Zhang J (2012) Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int J Neurosci 122:494–499. https://doi.org/10.3109/00207454.2012.686543
Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, McCall CE (2011a) NAD+−dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J Biol Chem 286:9856–9864. https://doi.org/10.1074/jbc.M110.196790
Liu Y et al (2011b) MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett 585:1963–1968. https://doi.org/10.1016/j.febslet.2011.05.029
Liu TF, Vachharajani VT, Yoza BK, McCall CE (2012) NAD+−dependent Sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem 287:25758–25769. https://doi.org/10.1074/jbc.M112.362343
Liu TF, Vachharajani V, Millet P, Bharadwaj MS, Molina AJ, McCall CE (2015a) Sequential actions of SIRT1-RELB-SIRT3 coordinate nuclear-mitochondrial communication during immunometabolic adaptation to acute inflammation and sepsis. J Biol Chem 290:396–408. https://doi.org/10.1074/jbc.M114.566349
Liu Z et al (2015b) AMP-activated protein kinase and glycogen synthase kinase 3β modulate the severity of Sepsis-induced lung injury. Mol Med 21:937–950. https://doi.org/10.2119/molmed.2015.00198
Liu P, Jing Y, Collie ND, Dean B, Bilkey DK, Zhang H (2016) Altered brain arginine metabolism in schizophrenia. Transl Psychiatry 6:e871. https://doi.org/10.1038/tp.2016.144
Loebel M et al (2014) Deficient EBV-specific B- and T-cell response in patients with chronic fatigue syndrome. PLoS One 9:e85387. https://doi.org/10.1371/journal.pone.0085387
Long X, Miano JM (2011) Transforming growth factor-β1 (TGF-β1) utilizes distinct pathways for the transcriptional activation of MicroRNA 143/145 in human coronary artery smooth muscle cells. J Biol Chem 286:30119–30129. https://doi.org/10.1074/jbc.M111.258814
Long D et al (2017) The oxidative state of cysteine thiol 144 regulates the SIRT6 glucose homeostat. Sci Rep 7:11005. https://doi.org/10.1038/s41598-017-11388-6
Lopez-Rivera E et al (2014) Inducible nitric oxide synthase drives mTOR pathway activation and proliferation of human melanoma by reversible nitrosylation of TSC2. Cancer Res 74:1067–1078. https://doi.org/10.1158/0008-5472.can-13-0588
Lucas K, Maes M (2013) Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol 48:190–204
Lucas K, Morris G, Anderson G, Maes M (2015) The toll-like receptor radical cycle pathway: a new drug target in immune-related chronic fatigue. CNS Neurol Disord Drug Targets 14:838–854
Ma J, Coarfa C, Qin X, Bonnen PE, Milosavljevic A, Versalovic J, Aagaard K (2014) mtDNA haplogroup and single nucleotide polymorphisms structure human microbiome communities. BMC Genomics 15:257. https://doi.org/10.1186/1471-2164-15-257
Ma Z et al (2016) MicroRNA regulatory pathway analysis identifies miR-142-5p as a negative regulator of TGF-β pathway via targeting SMAD3. Oncotarget 7:71504–71513. https://doi.org/10.18632/oncotarget.12229
Machale S, Lawrie S, Cavanagh J, Glabus M, Murray C, Goodwin G (2000) Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry 176:550–556
Maes M (2013) Inflammatory and oxidative and nitrosative stress cascades as new drug targets in myalgic encephalomyelitis and chronic fatigue syndrome. Mod Trends Pharmacopsychiatry 28:162–174. https://doi.org/10.1159/000343982
Maes M, Leunis JC (2014) Attenuation of autoimmune responses to oxidative specific epitopes, but not nitroso-adducts, is associated with a better clinical outcome in Myalgic encephalomyelitis/chronic fatigue syndrome. Neuro Endocrinol lett 35:577–585
Maes M, Mihaylova I, Leunis J (2006) Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuro Endocrinol Lett 27:615–621
Maes M, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E (2011) Increased plasma peroxides as a marker of oxidative stress in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med Sci Monit 17:SC11–SC15
Maes M, Twisk FN, Kubera M, Ringel K (2012a) Evidence for inflammation and activation of cell-mediated immunity in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): increased interleukin-1, tumor necrosis factor-alpha, PMN-elastase, lysozyme and neopterin. J Affect Disord 136:933–939. https://doi.org/10.1016/j.jad.2011.09.004
Maes M, Twisk FN, Ringel K (2012b) Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom 81:286–295. https://doi.org/10.1159/000336803
Maes M, Bosmans E, Kubera M (2015) Increased expression of activation antigens on CD8+ T lymphocytes in Myalgic encephalomyelitis/chronic fatigue syndrome: inverse associations with lowered CD19+ expression and CD4+/CD8+ ratio, but no associations with (auto)immune, leaky gut, oxidative and nitrosative stress biomarkers. Neuro Endocrinol Lett 36:439–446
Mailloux RJ, Jin X, Willmore WG (2014) Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions(). Redox Biol 2:123–139. https://doi.org/10.1016/j.redox.2013.12.011
Malfliet A et al. (2018) Cerebral blood flow and heart rate variability in chronic fatigue syndrome: a randomized cross-over study pain physician 21:E13-e24
Marshall HE, Hess DT, Stamler JS (2004) S-nitrosylation: physiological regulation of NF-kappaB. Proc Natl Acad Sci U S A 101:8841–8842. https://doi.org/10.1073/pnas.0403034101
Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB (2015) Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun 48:195–204. https://doi.org/10.1016/j.bbi.2015.03.015
Martinez-Martinez LA, Mora T, Vargas A, Fuentes-Iniestra M, Martinez-Lavin M (2014) Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studies. J Clin Rheumatol 20:146–150. https://doi.org/10.1097/rhu.0000000000000089
Masson GS, Nair AR, Dange RB, Silva-Soares PP, Michelini LC, Francis J (2015) Toll-like receptor 4 promotes autonomic dysfunction, inflammation and microglia activation in the hypothalamic paraventricular nucleus: role of endoplasmic reticulum stress. PLoS One 10:e0122850. https://doi.org/10.1371/journal.pone.0122850
McCall CE, Yoza B, Liu T, El Gazzar M (2010) Gene-specific epigenetic regulation in serious infections with systemic inflammation. J Innate Immun 2:395–405. https://doi.org/10.1159/000314077
McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B (2011) Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J Leukoc Biol 90:439–446. https://doi.org/10.1189/jlb.0211075
Meeus M, Ickmans K, Struyf F, Kos D, Lambrecht L, Willekens B, Cras P, Nijs J (2016) What is in a name? Comparing diagnostic criteria for chronic fatigue syndrome with or without fibromyalgia. Clin Rheumatol 35:191–203. https://doi.org/10.1007/s10067-014-2793-x
Merlo LM, Mandik-Nayak L (2016) IDO2: a pathogenic mediator of inflammatory autoimmunity. Clin Med Insights Pathol 9(Suppl 1):21–28
Merlo LM, DuHadaway JB, Grabler S, Prendergast GC, Muller AJ, Mandik-Nayak L (2016) IDO2 modulates T cell-dependent autoimmune responses through a B cell-intrinsic mechanism. J Immunol 196(11):4487–4497. https://doi.org/10.4049/jimmunol.1600141
Merlo LMF, Grabler S, DuHadaway JB, Pigott E, Manley K, Prendergast GC, Laury-Kleintop LD, Mandik-Nayak L (2017) Therapeutic antibody targeting of indoleamine-2,3-dioxygenase (IDO2) inhibits autoimmune arthritis. Clin Immunol 179:8–16. https://doi.org/10.1016/j.clim.2017.01.016
Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC (2007) Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res 67:7082–7087
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185:3190–3198. https://doi.org/10.4049/jimmunol.0903670
Mikirova N, Casciari J, Hunninghake R (2012) The assessment of the energy metabolism in patients with chronic fatigue syndrome by serum fluorescence emission. Altern Ther Health Med 18:36–40
Miller YI et al (2011) Oxidation-specific epitopes are danger associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res 108:235–248. https://doi.org/10.1161/CIRCRESAHA.110.223875
Miller AH, Haroon E, Raison CL, Felger JC (2013) Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30:297–306. https://doi.org/10.1002/da.22084
Millet P, McCall C, Yoza B (2013) RelB: an outlier in leukocyte biology. J Leukoc Biol 94:941–951. https://doi.org/10.1189/jlb.0513305
Milrad SF et al (2017) Poor sleep quality is associated with greater circulating pro-inflammatory cytokines and severity and frequency of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) symptoms in women. J Neuroimmunol 303:43–50. https://doi.org/10.1016/j.jneuroim.2016.12.008
Mitchell BM, Cook LG, Danchuk S, Puschett JB (2007) Uncoupled endothelial nitric oxide synthase and oxidative stress in a rat model of pregnancy-induced hypertension. Am J Hypertens 20:1297–1304. https://doi.org/10.1016/j.amjhyper.2007.08.007
Moens AL, Kass DA (2006) Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol 26:2439–2444. https://doi.org/10.1161/01.ATV.0000243924.00970.cb
Monro JA, Puri BK (2018) A molecular neurobiological approach to understanding the Aetiology of chronic fatigue syndrome (Myalgic encephalomyelitis or systemic exertion intolerance disease) with treatment implications. Mol Neurobiol. https://doi.org/10.1007/s12035-018-0928-9
Montezano AC, Touyz RM (2012) Reactive oxygen species and endothelial function--role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol 110:87–94. https://doi.org/10.1111/j.1742-7843.2011.00785.x
Montoya JG et al (2017) Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci U S A 114:E7150–e7158. https://doi.org/10.1073/pnas.1710519114
Morales-Alamo D, Calbet JAL (2016) AMPK signaling in skeletal muscle during exercise: role of reactive oxygen and nitrogen species. Free Radic Biol Med 98:68–77. https://doi.org/10.1016/j.freeradbiomed.2016.01.012
Morandini AC, Santos CF, Yilmaz O (2016) Role of epigenetics in modulation of immune response at the junction of host-pathogen interaction and danger molecule signaling. Pathog Dis 74(7). pii: ftw082. https://doi.org/10.1093/femspd/ftw082
Morris G, Berk M (2015) The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med 13:68
Morris G, Maes M (2013a) Case definitions and diagnostic criteria for myalgic encephalomyelitis and chronic fatigue syndrome: from clinical-consensus to evidence-based case definitions. Neuro Endocrinol Lett 34:185–199. https://doi.org/10.1186/1741-7015-11-205
Morris G, Maes M (2013b) Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med 11:205
Morris G, Maes M (2013c) A neuro-immune model of myalgic encephalomyelitis/chronic fatigue syndrome. Metab Brain Dis 28:523–540. https://doi.org/10.1007/s11011-012-9324-8
Morris G, Maes M (2014) Oxidative and nitrosative stress and immune-inflammatory pathways in patients with Myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr Neuropharmacol 12:168–185
Morris G, Anderson G, Galecki P, Berk M, Maes M (2013) A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior. BMC Med 11:64
Morris G, Berk M, Galecki P, Walder K, Maes M (2015a) The neuro-immune pathophysiology of central and peripheral fatigue in systemic immune-inflammatory and neuro-immune diseases. Mol Neurobiol 53:1195–1219. https://doi.org/10.1007/s12035-015-9090-9
Morris G, Berk M, Walder K, Maes M (2015b) Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med 13:28
Morris G, Berk M, Carvalho A, Caso JR, Sanz Y, Walder K, Maes M (2016a) The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and Neuroimmune disease. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0004-2
Morris G, Berk M, Carvalho AF, Caso JR, Sanz Y, Maes M (2016b) The role of microbiota and intestinal permeability in the pathophysiology of autoimmune and Neuroimmune processes with an emphasis on inflammatory bowel disease type 1 diabetes and chronic fatigue syndrome. Curr Pharm Des 22:6058–6075
Morris G, Berk M, Klein H, Walder K, Galecki P, Maes M (2016c) Nitrosative stress, Hypernitrosylation, and autoimmune responses to Nitrosylated proteins: new pathways in Neuroprogressive disorders including depression and chronic fatigue syndrome. Mol Neurobiol. https://doi.org/10.1007/s12035-016-9975-2
Morris G, Berk M, Walder K, Maes M (2016d) The putative role of viruses, Bacteria, and chronic fungal biotoxin exposure in the genesis of intractable fatigue accompanied by cognitive and physical. Disabil Mol Neurobiol 53:2550–2571. https://doi.org/10.1007/s12035-015-9262-7
Morris G, Carvalho AF, Anderson G, Galecki P, Maes M (2016e) The many neuroprogressive actions of tryptophan catabolites (TRYCATs) that may be associated with the pathophysiology of neuro-immune disorders. Curr Pharm Des 22:963–977
Morris G, Anderson G, Maes M (2017a) Hypothalamic-pituitary-adrenal hypofunction in Myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and Nitrosative pathways. Mol Neurobiol 54:6806–6819. https://doi.org/10.1007/s12035-016-0170-2
Morris G, Berk M, Puri BK (2017b) A comparison of neuroimaging abnormalities in multiple sclerosis, major depression and chronic fatigue syndrome (Myalgic encephalomyelitis): is there a common cause? Mol Neurobiol. https://doi.org/10.1007/s12035-017-0598-z
Morris G, Walder K, Carvalho AF, Tye SJ, Lucas K, Berk M, Maes M (2017c) The role of hypernitrosylation in the pathogenesis and pathophysiology of neuroprogressive diseases. Neurosci Biobehav Rev. https://doi.org/10.1016/j.neubiorev.2017.07.017
Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, Berk M (2017d) A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev 74:1–20. https://doi.org/10.1016/j.neubiorev.2017.01.014
Murray CI, Uhrigshardt H, O'Meally RN, Cole RN, Van Eyk JE (2012) Identification and quantification of S-Nitrosylation by cysteine reactive tandem mass tag switch assay. Mol Cell Proteomics 11:M111.013441. https://doi.org/10.1074/mcp.M111.013441
Nagatsu T, Sawada M (2006) Cellular and molecular mechanisms of Parkinson's disease: neurotoxins, causative genes, and inflammatory cytokines. Cell Mol Neurobiol 26:781–802. https://doi.org/10.1007/s10571-006-9061-9
Nahid MA, Pauley KM, Satoh M, Chan EK (2009) miR-146a is critical for endotoxin-induced tolerance: implication In Innate Immunity. J Biol Chem 284:34590–34599. https://doi.org/10.1074/jbc.M109.056317
Nahid MA, Satoh M, Chan EK (2011) MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol 8:388–403. https://doi.org/10.1038/cmi.2011.26
Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D (2013) Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation 10:142. https://doi.org/10.1186/1742-2094-10-142
Nakahira K, Hisata S, Choi AMK (2015) The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal 23:1329–1350. https://doi.org/10.1089/ars.2015.6407
Nakamura T, Cieplak P, Cho D-H, Godzik A, Lipton SA (2010) S-Nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion 10:573–578. https://doi.org/10.1016/j.mito.2010.04.007
Nakazawa H et al (2017) iNOS as a driver of inflammation and apoptosis in mouse skeletal muscle after burn injury: possible involvement of Sirt1 S-Nitrosylation-mediated acetylation of p65 NF-κB and p53. PLoS One 12:e0170391. https://doi.org/10.1371/journal.pone.0170391
Naschitz JE, Yeshurun D, Rosner I (2004) Dysautonomia in chronic fatigue syndrome: facts, hypotheses, implications. Med Hypotheses 62:203–206. https://doi.org/10.1016/s0306-9877(03)00331-1
Naschitz J, Fields M, Isseroff H, Sharif D, Sabo E, Rosner I (2006) Shortened QT interval: a distinctive feature of the dysautonomia of chronic fatigue syndrome. J Electrocardiol 39:389–394. https://doi.org/10.1016/j.jelectrocard.2005.10.014
Natelson B, Haghighi M, Ponzio N (2002) Evidence for the presence of immune dysfunction in chronic fatigue syndrome. Clin Diagn Lab Immunol 9:747–752
Naviaux RK (2018) Metabolic features and regulation of the healing cycle - a new model for chronic disease pathogenesis and treatment. Mitochondrion pii: S1567-7249(18)30105-3. https://doi.org/10.1016/j.mito.2018.08.001
Naviaux RK et al (2016) Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci 113:E5472–E5480. https://doi.org/10.1073/pnas.1607571113
Newton JL, Okonkwo O, Sutcliffe K, Seth A, Shin J, Jones DE (2007) Symptoms of autonomic dysfunction in chronic fatigue syndrome. Qjm 100:519–526. https://doi.org/10.1093/qjmed/hcm057
Nguyen CB et al (2017) Whole blood gene expression in adolescent chronic fatigue syndrome: an exploratory cross-sectional study suggesting altered B cell differentiation and survival. J Transl Med 15:102. https://doi.org/10.1186/s12967-017-1201-0
Nijhof LN, Nijhof SL, Bleijenberg G, Stellato RK, Kimpen JLL, Pol HEH, van de Putte EM (2016) The impact of chronic fatigue syndrome on cognitive functioning in adolescents. Eur J Pediatr 175:245–252. https://doi.org/10.1007/s00431-015-2626-1
Nijs J, Crombez G, Meeus M, Knoop H, Damme SV, Cauwenbergh V, Bleijenberg G (2012) Pain in patients with chronic fatigue syndrome: time for specific pain treatment? Pain Physician 15:E677–E686
Noda M (2016) Dysfunction of glutamate receptors in microglia may cause neurodegeneration. Curr Alzheimer Res 13:381–386
Nolan Y et al (2005) Role of Interleukin-4 in regulation of age-related inflammatory changes in the Hippocampus. J Biol Chem 280:9354–9362. https://doi.org/10.1074/jbc.M412170200
Novak EA, Mollen KP (2015) Mitochondrial dysfunction in inflammatory bowel disease. Front Cell Dev Biol 3:62. https://doi.org/10.3389/fcell.2015.00062
Nsiah-Sefaa A, McKenzie M (2016) Combined defects in oxidative phosphorylation and fatty acid β-oxidation in mitochondrial disease. Biosci Rep 36:e00313. https://doi.org/10.1042/BSR20150295
Numajiri N et al (2011) On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN). Proc Natl Acad Sci U S A 108:10349–10354. https://doi.org/10.1073/pnas.1103503108
Ocon AJ (2013) Caught in the thickness of brain fog: exploring the cognitive symptoms of chronic fatigue syndrome. Front Physiol 4:63. https://doi.org/10.3389/fphys.2013.00063
Ogawa H et al (2003) Mechanisms of endotoxin tolerance in human intestinal microvascular endothelial cells. J Immunol 170:5956–5964. https://doi.org/10.4049/jimmunol.170.12.5956
Ogawa K, Hirooka Y, Kishi T, Sunagawa K (2011) Brain AT1 receptor activates the sympathetic nervous system through toll-like receptor 4 in mice with heart failure. J Cardiovasc Pharmacol 58:543–549. https://doi.org/10.1097/FJC.0b013e31822e6b40
Oh CK et al (2017) S-Nitrosylation of PINK1 attenuates PINK1/Parkin-dependent Mitophagy in hiPSC-based Parkinson's disease models. Cell Rep 21:2171–2182. https://doi.org/10.1016/j.celrep.2017.10.068
Okada T, Tanak M, Kuratsune H, Watanabe Y, Sadato N (2004) Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol 4:14
Okamoto S, Lipton SA (2015) S-Nitrosylation in neurogenesis and neuronal development. Biochim Biophys Acta 1850:1588–1593. https://doi.org/10.1016/j.bbagen.2014.12.013
Okun E et al (2014) Toll-like receptors 2 and 4 modulate autonomic control of heart rate and energy metabolism. Brain Behav Immun 36:90–100. https://doi.org/10.1016/j.bbi.2013.10.013
Ortiz G, Pacheco-Moises F, Bitzer-Quintero O, Ramirez-Anguiano A, Flores-Alvarado L, Ramirez-Ramirez V (2013) Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol 2013:708659
Ouwendijk WJ, Laing KJ, Verjans GM, Koelle DM (2013) T-cell immunity to human alphaherpesviruses. Curr Opin Virol 3:452–460. https://doi.org/10.1016/j.coviro.2013.04.004
Ozawa K et al (2013) S-nitrosylation regulates mitochondrial quality control via activation of parkin. Sci Rep 3:2202. https://doi.org/10.1038/srep02202 https://www.nature.com/articles/srep02202#supplementary-information
Pachot A et al (2005) Longitudinal study of cytokine and immune transcription factor mRNA expression in septic shock. Clin Immunol 114:61–69. https://doi.org/10.1016/j.clim.2004.08.015
Pallotta MT et al (2011) Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 12:870–878. https://doi.org/10.1038/ni.2077
Pan H, Ding E, Hu M, Lagoo AS, Datto MB, Lagoo-Deenadayalan SA (2010) SMAD4 is required for development of maximal endotoxin tolerance. J Immunol (Baltimore, Md : 1950) 184:5502–5509. https://doi.org/10.4049/jimmunol.0901601
Pan A et al (2014) Phenotype and functions of natural killer cells in septic patients and its clinical significance. Zhonghua wei zhong bing ji jiu yi xue 26:827–831. https://doi.org/10.3760/cma.j.issn.2095-4352.2014.11.012
Park SJ, Cheon EJ, Lee MH, Kim HA (2013) MicroRNA-127-5p regulates matrix metalloproteinase 13 expression and interleukin-1beta-induced catabolic effects in human chondrocytes. Arthritis Rheum 65:3141–3152. https://doi.org/10.1002/art.38188
Park DW, Jiang S, Liu Y, Siegal GP, Inoki K, Abraham E, Zmijewski JW (2014) GSK3beta-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 307:L735–L745. https://doi.org/10.1152/ajplung.00165.2014
Parker LC, Jones EC, Prince LR, Dower SK, Whyte MK, Sabroe I (2005) Endotoxin tolerance induces selective alterations in neutrophil function. J Leukoc Biol 78:1301–1305. https://doi.org/10.1189/jlb.0405236
Patrick Neary J, Roberts AD, Leavins N, Harrison MF, Croll JC, Sexsmith JR (2008) Prefrontal cortex oxygenation during incremental exercise in chronic fatigue syndrome. Clin Physiol Funct Imaging 28:364–372. https://doi.org/10.1111/j.1475-097X.2008.00822.x
Paulsen CE, Carroll KS (2010) Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol 5:47–62. https://doi.org/10.1021/cb900258z
Pena OM, Pistolic J, Raj D, Fjell CD, Hancock REW (2011) Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol 186:7243–7254. https://doi.org/10.4049/jimmunol.1001952
Penna C, Angotti C, Pagliaro P (2014) Protein S-nitrosylation in preconditioning and postconditioning. Exp Biol Med (Maywood) 239:647–662. https://doi.org/10.1177/1535370214522935
Perrin R, Embleton K, Pentreath VW, Jackson A (2010) Longitudinal MRI shows no cerebral abnormality in chronic fatigue syndrome. Br J Radiol 83:419–423. https://doi.org/10.1259/bjr/85621779
Peterson PK, Sirr SA, Grammith FC, Schenck CH, Pheley AM, Hu S, Chao CC (1994) Effects of mild exercise on cytokines and cerebral blood flow in chronic fatigue syndrome patients. Clin Diagn Lab Immunol 1:222–226
Peterson D, Brenu EW, Gottschalk G, Ramos S, Nguyen T, Staines D (2015) Marshall-Gradisnik S (2015) cytokines in the cerebrospinal fluids of patients with chronic fatigue syndrome/myalgic encephalomyelitis. Mediat Inflamm. https://doi.org/10.1155/2015/929720
Petty RD, McCarthy NE, Le Dieu R, Kerr JR (2016) MicroRNAs hsa-miR-99b, hsa-miR-330, hsa-miR-126 and hsa-miR-30c: potential diagnostic biomarkers in natural killer (NK) cells of patients with chronic fatigue syndrome (CFS)/ Myalgic encephalomyelitis (ME). PLoS One 11:e0150904. https://doi.org/10.1371/journal.pone.0150904
Piantadosi CA (2012) Regulation of mitochondrial processes by protein S-nitrosylation. Biochim Biophys Acta 1820:712–721. https://doi.org/10.1016/j.bbagen.2011.03.008
Piccinini AM, Midwood KS (2010) DAMPening inflammation by modulating TLR signalling. Mediat Inflamm 2010. https://doi.org/10.1155/2010/672395
Pillai VB, Sundaresan NR, Gupta MP (2014) Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ Res 114:368–378. https://doi.org/10.1161/circresaha.113.300536
Piraino B, Vollmer-Conna U, Lloyd AR (2012) Genetic associations of fatigue and other symptom domains of the acute sickness response to infection. Brain Behav Immun 26:552–558. https://doi.org/10.1016/j.bbi.2011.12.009
Price NL et al (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. https://doi.org/10.1016/j.cmet.2012.04.003
Prieto J, Subira ML, Castilla A, Serrano M (1989) Naloxone-reversible monocyte dysfunction in patients with chronic fatigue syndrome. Scand J Immunol 30:13–20
Prieto-Domínguez N et al (2016) Melatonin-induced increase in sensitivity of human hepatocellular carcinoma cells to sorafenib is associated with reactive oxygen species production and mitophagy. J Pineal Res 61:396–407. https://doi.org/10.1111/jpi.12358
Puddu A, Sanguineti R, Montecucco F, Viviani GL (2014) Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat Inflamm 2014:1–9. https://doi.org/10.1155/2014/162021
Puri BK, Counsell S, Zaman R, Main J, Collins A, Hajnal J, Davey N (2002) Relative increase in choline in the occipital cortex in chronic fatigue syndrome. Acta Psychiatr Scand 106:224–226
Puri BK et al (2012) Regional grey and white matter volumetric changes in myalgic encephalomyelitis (chronic fatigue syndrome): a voxel-based morphometry 3 T MRI study. Br J Radiol 85:e270–e273. https://doi.org/10.1259/bjr/93889091
Qu Q, Zeng F, Liu X, Wang QJ, Deng F (2016) Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis 7:e2226. https://doi.org/10.1038/cddis.2016.132
Quinn EM, Wang J, Redmond HP (2012) The emerging role of microRNA in regulation of endotoxin tolerance. J Leukoc Biol 91:721–727. https://doi.org/10.1189/jlb.1111571
Racciatti D et al (2004) Study of immune alterations in patients with chronic fatigue syndrome with different etiologies. Int J Immunopathol Pharmacol 17:57–62. https://doi.org/10.1177/03946320040170s210
Raj D et al (2017) Increased White matter inflammation in aging- and Alzheimer’s disease. Brain Front Mol Neurosci 10:206. https://doi.org/10.3389/fnmol.2017.00206
Raker VK, Domogalla MP, Steinbrink K (2015) Tolerogenic dendritic cells for regulatory T cell induction in man. Front Immunol 6:569. https://doi.org/10.3389/fimmu.2015.00569
Ramos S et al (2016) Regulatory T, natural killer T and gammadelta T cells in multiple sclerosis and chronic fatigue syndrome/myalgic encephalomyelitis: a comparison. Asian Pac J Allergy Immunol 34:300–305. https://doi.org/10.12932/ap0733
Rautanen A et al (2015) Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. Lancet Respir Med 3:53–60. https://doi.org/10.1016/s2213-2600(14)70290-5
Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P (2011) Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev 67:103–118. https://doi.org/10.1016/j.brainresrev.2010.11.004
Reuter S, Gupta S, Chaturvedi M, Aggarwal B (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49:1603–1616
Reynaert NL et al (2004) Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A 101:8945–8950. https://doi.org/10.1073/pnas.0400588101
Riccio P, Rossano R (2015) Nutrition Facts in Multiple Sclerosis. ASN Neuro 7:1759091414568185. https://doi.org/10.1177/1759091414568185
Richards RS, Wang L, Jelinek H (2007) Erythrocyte oxidative damage in chronic fatigue syndrome. Arch Med Res 38:94–98. https://doi.org/10.1016/j.arcmed.2006.06.008
Rigato O, Salomao R (2003) Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock (Augusta, Ga) 19:113–116
Rimbaut S, Van Gutte C, Van Brabander L, Vanden Bossche L (2016) Chronic fatigue syndrome - an update. Acta Clin Belg 17:1–8
Rittirsch D, Flierl MA, Ward PA (2008) Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8:776–787. https://doi.org/10.1038/nri2402
Riverol M et al (2012) Systemic inflammatory markers, cognition and brain structure among cognitively Normal elderly (P02.061). Neurology 78:P02.061
Robel S et al (2015) Reactive Astrogliosis causes the development of spontaneous seizures. J Neurosci 35:3330–3345. https://doi.org/10.1523/JNEUROSCI.1574-14.2015
Robinson LJ, Durham J, MacLachlan LL, Newton JL (2015) Autonomic function in chronic fatigue syndrome with and without painful temporomandibular disorder. Fatigue 3:205–219. https://doi.org/10.1080/21641846.2015.1091152
Roerink ME, Knoop H, Bronkhorst EM, Mouthaan HA, Hawinkels LJAC, Joosten LAB, van der Meer JWM (2017) Cytokine signatures in chronic fatigue syndrome patients: a case control study and the effect of anakinra treatment. J Transl Med 15:267. https://doi.org/10.1186/s12967-017-1371-9
Rogers GL, Herzog RW (2014) One MicroRNA controls both angiogenesis and TLR-mediated innate immunity to nucleic acids. Mol Ther 22:249–250. https://doi.org/10.1038/mt.2013.299
Ross AJ, Medow MS, Rowe PC, Stewart JM (2013) What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res 23:305–311. https://doi.org/10.1007/s10286-013-0212-z
Russell L et al (2016) Illness progression in chronic fatigue syndrome: a shifting immune baseline. BMC Immunol 17:1–11. https://doi.org/10.1186/s12865-016-0142-3
Rutherford G, Manning P, Newton JL (2016) Understanding muscle dysfunction in chronic fatigue syndrome. J Aging Res 2016:2497348. https://doi.org/10.1155/2016/2497348
Saiki T, Kawai T, Morita K, Ohta M, Saito T, Rokutan K, Ban N (2008) Identification of marker genes for differential diagnosis of chronic fatigue syndrome. Mol Med (Cambridge, Mass) 14:599–607. https://doi.org/10.2119/2007-00059.Saiki
Salazar F, Awuah D, Negm OH, Shakib F, Ghaemmaghami AM (2017) The role of indoleamine 2,3-dioxygenase-aryl hydrocarbon receptor pathway in the TLR4-induced tolerogenic phenotype in human DCs. Sci Rep 7:43337. https://doi.org/10.1038/srep43337
Sandiego CM et al (2015) Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci 112:12468–12473. https://doi.org/10.1073/pnas.1511003112
Sankowski R, Mader S, Valdés-Ferrer SI (2015) Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci 9:28. https://doi.org/10.3389/fncel.2015.00028
Santamarina-Perez P, Eiroa-Orosa FJ, Rodriguez-Urrutia A, Qureshi A, Alegre J (2014) Neuropsychological impairment in female patients with chronic fatigue syndrome: a preliminary study. Appl Neuropsychol Adult 21:120–127. https://doi.org/10.1080/09084282.2013.771264
Sarti P, Giuffre A, Forte E, Mastronicola D, Barone MC, Brunori M (2000) Nitric oxide and cytochrome c oxidase: mechanisms of inhibition and NO degradation. Biochem Biophys Res Commun 274:183–187. https://doi.org/10.1006/bbrc.2000.3117
Sarti P, Arese M, Giuffre A (2003a) The molecular mechanisms by which nitric oxide controls mitochondrial complex IV. Ital J Biochem 52:37–42
Sarti P, Giuffre A, Barone M, Forte E, Mastronicola D, Brunori M (2003b) Nitric oxide and cytochrome oxidase: reaction mechanisms from the enzyme to the cell. Free Radic Biol Med 34:509–520
Sarti P, Arese M, Forte E, Giuffre A, Mastronicola D (2012a) Mitochondria and nitric oxide: chemistry and pathophysiology. Adv Exp Med Biol 942:75–92. https://doi.org/10.1007/978-94-007-2869-1_4
Sarti P, Forte E, Giuffre A, Mastronicola D, Magnifico M, Arese M (2012b) The chemical interplay between nitric oxide and mitochondrial cytochrome c oxidase: reactions, effectors and pathophysiology. Int J Cell Biol 2012:571067
Sartori AC, Vance DE, Slater LZ, Crowe M (2012) The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J Neurosci Nurs 44:206–217. https://doi.org/10.1097/JNN.0b013e3182527690
Schultz HD (2009) Nitric oxide regulation of autonomic function in heart failure. Curr Heart Fail Rep 6:71–80
Schwartz R, Garada B, Komaroff A, Tice H, Gleit M, Jolesz F, Holman B (1994) Detection of intracranial abnormalities in patients with chronic fatigue syndrome: comparison of MR imaging and SPECT. AJR Am J Roentgenol 162:935–941
Sebastian C et al (2012) The histone deacetylase SIRT6, a critical modulator of metabolism and tumorigenesis. BMC Proc 6:O17. https://doi.org/10.1186/1753-6561-6-s3-o17
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721–732. https://doi.org/10.1038/nrc1187
Semenza GL (2011) Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol 76:347–353. https://doi.org/10.1101/sqb.2011.76.010678
Semenza GL, Jiang B-H, Leung SW, Passantino R, Concordet J-P, Maire P, Giallongo A (1996) Hypoxia response elements in the aldolase a, enolase 1, and lactate dehydrogenase a gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271:32529–32537. https://doi.org/10.1074/jbc.271.51.32529
Seruga B, Zhang H, Bernstein LJ, Tannock IF (2008) Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 8:887–899
Shan L, Siliciano RF (2014) Unraveling the relationship between microbial translocation and systemic immune activation in HIV infection. J Clin Investig 124:2368–2371. https://doi.org/10.1172/jci75799
Shan ZY, Kwiatek R, Burnet R, Del Fante P, Staines DR, Marshall-Gradisnik SM, Barnden LR (2016) Progressive brain changes in patients with chronic fatigue syndrome: a longitudinal MRI study. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25283
Shanks L, Jason LA, Evans M, Brown A (2013) Cognitive impairments associated with CFS and POTS. Front Physiol 4:113. https://doi.org/10.3389/fphys.2013.00113
Sharpe M, Archard L, Banatvala J, Borysiewicz L, Clare A, David A (1991) A report-chronic fatigue syndrome: guidelines for research. J R Soc Med 84:118–121
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685–700
Shimosako N, Kerr JR (2014) Use of single-nucleotide polymorphisms (SNPs) to distinguish gene expression subtypes of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Clin Pathol. https://doi.org/10.1136/jclinpath-2014-202597
Shinozaki S et al (2014) Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci Signal 7:ra106. https://doi.org/10.1126/scisignal.2005375
Shiva S et al (2007) Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204:2089–2102. https://doi.org/10.1084/jem.20070198
Singer M (2007) Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med 35:S441–S448. https://doi.org/10.1097/01.ccm.0000278049.48333.78
Singer M (2008) Cellular dysfunction in sepsis. Clin Chest Med 29:655–660, viii-ix. https://doi.org/10.1016/j.ccm.2008.06.003
Singer M (2017) Critical illness and flat batteries. Critical Care (London, England) 21:309. https://doi.org/10.1186/s13054-017-1913-9
Singh Y et al (2013) Mycobacterium tuberculosis controls MicroRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity. J Biol Chem 288:5056–5061. https://doi.org/10.1074/jbc.C112.439778
Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT (2014) Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 8. https://doi.org/10.3389/fnins.2014.00315
Sinistro A et al (2008) Downregulation of CD40 ligand response in monocytes from sepsis patients. Clin Vaccine Immunol 15:1851–1858. https://doi.org/10.1128/cvi.00184-08
Sly LM, Rauh MJ, Kalesnikoff J, Song CH, Krystal G (2004) LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity 21:227–239. https://doi.org/10.1016/j.immuni.2004.07.010
Smelaya TV et al (2016) Genetic dissection of host immune response in pneumonia development and progression. Sci Rep 6:35021. https://doi.org/10.1038/srep35021
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119. https://doi.org/10.1007/s00401-009-0619-8
Song SB, Jang S-Y, Kang HT, Wei B, Jeoun U-W, Yoon GS, Hwang ES (2017) Modulation of Mitochondrial Membrane Potential and ROS Generation by Nicotinamide in a Manner Independent of SIRT1 and Mitophagy. Mol Cell 40:503–514. https://doi.org/10.14348/molcells.2017.0081
Sonneville R et al (2013) Understanding brain dysfunction in sepsis. Ann Intensive Care 3:15. https://doi.org/10.1186/2110-5820-3-15
Srivastav S, Saha A, Barua J, Ukil A, Das PK (2015) IRAK-M regulates the inhibition of TLR-mediated macrophage immune response during late in vitro Leishmania donovani infection. Eur J Immunol 45:2787–2797. https://doi.org/10.1002/eji.201445336
Srivastava A, Mannam P (2015) Warburg revisited: lessons for innate immunity and sepsis. Front Physiol 6:70. https://doi.org/10.3389/fphys.2015.00070
Standiford TJ, Kuick R, Bhan U, Chen J, Newstead M, Keshamouni VG (2011) TGF-beta-induced IRAK-M expression in tumor-associated macrophages regulates lung tumor growth. Oncogene 30:2475–2484. https://doi.org/10.1038/onc.2010.619
Stiehm M, Peters K, Wiesmuller KH, Bufe A, Peters M (2013) A novel synthetic lipopeptide is allergy-protective by the induction of LPS-tolerance. Clin Exp Allergy 43:785–797. https://doi.org/10.1111/cea.12116
Stormorken E, Jason LA, Kirkevold M (2015) Fatigue in adults with post-infectious fatigue syndrome: a qualitative content analysis. BMC Nurs 14:64. https://doi.org/10.1186/s12912-015-0115-5
Straus SE, Fritz S, Dale JK, Gould B, Strober W (1993) Lymphocyte phenotype and function in the chronic fatigue syndrome. J Clin Immunol 13:30–40
Strother RK et al (2016) Polymicrobial Sepsis diminishes dendritic cell numbers and function directly contributing to impaired primary CD8 T cell responses in vivo. J Immunol (Baltimore, Md : 1950) 197:4301–4311. https://doi.org/10.4049/jimmunol.1601463
Stuckey MI, Petrella RJ (2013) Heart rate variability in type 2 diabetes mellitus. Crit Rev Biomed Eng 41:137–147
Studer V et al (2017) Heart rate variability is differentially altered in multiple sclerosis: implications for acute, worsening and progressive disability. Mult Scler J Exp Transl Clin 3:2055217317701317. https://doi.org/10.1177/2055217317701317
Sun J, Murphy E (2010) Protein S-nitrosylation and cardioprotection. Circ Res 106:285–296. https://doi.org/10.1161/circresaha.109.209452
Sun J, Steenbergen C, Murphy E (2006) S-Nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal 8:1693–1705. https://doi.org/10.1089/ars.2006.8.1693
Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E (2007) Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res 101:1155–1163. https://doi.org/10.1161/circresaha.107.155879
Suzuki T et al (2013) Inhibition of AMPK catabolic action by GSK3. Mol Cell 50:407–419. https://doi.org/10.1016/j.molcel.2013.03.022
Swanink CMA, Melchers WJG, van der Meer JWM, Vercoulen JHMM, Bleijenberg G, Fennis JFM, Galama JMD (1994) Enteroviruses and the chronic fatigue syndrome. Clin Infect Dis 19:860–864. https://doi.org/10.1093/clinids/19.5.860
Szabó C, Ischiropoulos H, Radi R (2007) Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6:662–680. https://doi.org/10.1038/nrd2222
Takeuchi H et al (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281:21362–21368. https://doi.org/10.1074/jbc.M600504200
Tan MS et al (2013) NLRP3 polymorphisms are associated with late-onset Alzheimer's disease in Han Chinese. J Neuroimmunol 265:91–95. https://doi.org/10.1016/j.jneuroim.2013.10.002
Tanaka M et al (2006) Reduced responsiveness is an essential feature of chronic fatigue syndrome: a fMRI study. BMC Neurol 6. https://doi.org/10.1186/1471-2377-6-9
Tateishi Y, Oda S, Nakamura M, Watanabe K, Kuwaki T, Moriguchi T, Hirasawa H (2007) Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock (Augusta, Ga) 28:549–553. https://doi.org/10.1097/shk.0b013e3180638d1
Testa U, Pelosi E, Castelli G, Labbaye C (2017) miR-146 and miR-155: two key modulators of immune response and tumor development. Non-Coding RNA 3 doi:https://doi.org/10.3390/ncrna3030022
Thambirajah AA, Sleigh K, Stiver HG, Chow AW (2008) Differential heat shock protein responses to strenuous standardized exercise in chronic fatigue syndrome patients and matched healthy controls. Clin Invest Med 31:E319–E327
Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ (2007) Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component. RelB Am J Pathol 170:855–864. https://doi.org/10.2353/ajpath.2007.060391
Theoharides TC, Stewart JM, Hatziagelaki E, Kolaitis G (2015) Brain "fog," inflammation and obesity: key aspects of neuropsychiatric disorders improved by luteolin. Front Neurosci 9:225. https://doi.org/10.3389/fnins.2015.00225
Tian T, Wang Z, Zhang J (2017) Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxidative Med Cell Longev 2017:18. https://doi.org/10.1155/2017/4535194
Tirelli V et al (1993) Clinical and immunologic study of 205 patients with chronic fatigue syndrome: a case series from Italy. Arch Intern Med 153(116–117):120
Tirelli U, Marotta G, Improta S, Pinto A (1994) Immunological abnormalities in patients with chronic fatigue syndrome. Scand J Immunol 40:601–608
Toda N, Okamura T (2012) Cerebral blood flow regulation by nitric oxide in Alzheimer's disease. J Alzheimers Dis 32:569–578. https://doi.org/10.3233/jad-2012-120670
Tomas C, Newton J, Watson S (2013) A review of hypothalamic-pituitary-adrenal Axis function in chronic fatigue syndrome. ISRN Neuroscience 2013:8. https://doi.org/10.1155/2013/784520
Tomas C, Brown A, Strassheim V, Elson J, Newton J, Manning P (2017) Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS One 12:e0186802. https://doi.org/10.1371/journal.pone.0186802
Tomic S, Brkic S, Maric D, Mikic AN (2012) Lipid and protein oxidation in female patients with chronic fatigue syndrome. Arch Med Sci 8:886–891. https://doi.org/10.5114/aoms.2012.31620
Tóth E et al (2017) Gray Matter Atrophy Is Primarily Related to Demyelination of Lesions in Multiple Sclerosis: A Diffusion Tensor Imaging MRI Study. Front Neuroanat 11. https://doi.org/10.3389/fnana.2017.00023
Trewin AJ, Berry BJ, Wojtovich AP (2018) Exercise and mitochondrial dynamics: keeping in shape with ROS and AMPK. Antioxidants (Basel, Switzerland) 7. https://doi.org/10.3390/antiox7010007
Tripathy D, Chakraborty J, Mohanakumar KP (2015) Antagonistic pleiotropic effects of nitric oxide in the pathophysiology of Parkinson’s disease. Free Radic Res 49. https://doi.org/10.3109/10715762.2015.1045505
Tseng AH, Shieh SS, Wang DL (2013) SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med 63:222–234. https://doi.org/10.1016/j.freeradbiomed.2013.05.002
Uchida K (2013) Redox-derived damage-associated molecular patterns: ligand function of lipid peroxidation adducts. Redox Biol 1:94–96. https://doi.org/10.1016/j.redox.2012.12.005
Ulleryd MA, Prahl U, Borsbo J, Schmidt C, Nilsson S, Bergstrom G, Johansson ME (2017) The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS One 12:e0174974. https://doi.org/10.1371/journal.pone.0174974
Vachharajani V, Liu T, McCall CE (2014) Epigenetic coordination of acute systemic inflammation: potential therapeutic targets. Expert Rev Clin Immunol 10:1141–1150. https://doi.org/10.1586/1744666X.2014.943192
Vachharajani VT, Liu T, Wang X, Hoth JJ, Yoza BK, McCall CE (2016) Sirtuins link inflammation and metabolism. J Immunol Res 2016:8167273. https://doi.org/10.1155/2016/8167273
Van Cauwenbergh D, Nijs J, Kos D, Van Weijnen L, Struyf F, Meeus M (2014) Malfunctioning of the autonomic nervous system in patients with chronic fatigue syndrome: a systematic literature review. Eur J Clin Investig 44:516–526. https://doi.org/10.1111/eci.12256
van Eden W, Spiering R, Broere F, van der Zee R (2012) A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress Chaperones 17:281–292. https://doi.org/10.1007/s12192-011-0311-5
van Eden W, Jansen MAA, Ludwig I, van Kooten P, van der Zee R, Broere F (2017) The enigma of heat shock proteins in immune tolerance. Front Immunol 8:1599. https://doi.org/10.3389/fimmu.2017.01599
van Uden P, Kenneth Niall S, Rocha S (2008) Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem J 412:477–484. https://doi.org/10.1042/BJ20080476
van’t Veer C et al (2007) Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J Immunol (Baltimore, Md : 1950) 179:7110–7120
Vangeel E et al (2015) Chronic fatigue syndrome and DNA Hypomethylation of the glucocorticoid receptor gene promoter 1F region: associations with HPA Axis hypofunction and childhood trauma. Psychosom Med 77:853–862. https://doi.org/10.1097/psy.0000000000000224
Vangeel EB et al (2018) Glucocorticoid receptor DNA methylation and childhood trauma in chronic fatigue syndrome patients. J Psychosom Res 104:55–60. https://doi.org/10.1016/j.jpsychores.2017.11.011
Vaziri H et al (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107:149–159
Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F (2008) Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun 375:331–335. https://doi.org/10.1016/j.bbrc.2008.07.156
Vogel CFA et al (2013) Aryl hydrocarbon receptor signaling regulates NF-κB RelB activation during dendritic-cell differentiation. Immunol Cell Biol 91:568–575. https://doi.org/10.1038/icb.2013.43
Vollmer-Conna U, Aslakson E, White PD (2006) An empirical delineation of the heterogeneity of chronic unexplained fatigue in women. Pharmacogenomics 7. https://doi.org/10.2217/14622416.7.3.355
Vollmer-Conna U et al (2008) Cytokine polymorphisms have a synergistic effect on severity of the acute sickness response to infection. Clin Infect Dis 47:1418–1425. https://doi.org/10.1086/592967
Wanders RJA, Ruiter JPN, Ijlst L, Waterham HR, Houten SM (2010) The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab Dis 33:479–494. https://doi.org/10.1007/s10545-010-9104-8
Wang F, Xia ZF, Chen XL, Jia YT, Wang YJ, Ma B (2009) Angiotensin II type-1 receptor antagonist attenuates LPS-induced acute lung injury. Cytokine 48:246–253. https://doi.org/10.1016/j.cyto.2009.08.001
Wang J, Sun C, Zheng Y, Pan H, Zhou Y, Fan Y (2014) The effective mechanism of the polysaccharides from Panax ginseng on chronic fatigue syndrome. Arch Pharm Res 37:530–538. https://doi.org/10.1007/s12272-013-0235-y
Wenceslau CF, CG MC, Szasz T, Spitler K, Goulopoulou S, Webb RC, Working Group on DiCD (2014) Mitochondrial damage-associated molecular patterns and vascular function(). Eur Heart J 35:1172–1177. https://doi.org/10.1093/eurheartj/ehu047
White PD (2007) What causes prolonged fatigue after infectious mononucleosis—and does it tell us anything about chronic fatigue syndrome? J Infect Dis 196:4–5. https://doi.org/10.1086/518615
White PD et al (2011) Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet 377:823–836. https://doi.org/10.1016/S0140-6736(11)60096-2
White AT, Light AR, Hughen RW, Vanhaitsma TA, Light KC (2012) Differences in metabolite-detecting, adrenergic, and immune gene expression after moderate exercise in patients with chronic fatigue syndrome, patients with multiple sclerosis, and healthy controls. Psychosom Med 74:46–54. https://doi.org/10.1097/PSY.0b013e31824152ed
Wichers MC, Maes M (2004) The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-α-induced depression. J Psychiatry Neurosci 29:11–17
Wiersinga WJ, van't Veer C, van den Pangaart PS, Dondorp AM, Day NP, Peacock SJ, van der Poll T (2009) Immunosuppression associated with interleukin-1R-associated-kinase-M upregulation predicts mortality in gram-negative sepsis (melioidosis). Crit Care Med 37:569–576. https://doi.org/10.1097/CCM.0b013e318194b1bf
Wiersinga WJ, Leopold SJ, Cranendonk DR, van der Poll T (2014) Host innate immune responses to sepsis. Virulence 5:36–44. https://doi.org/10.4161/viru.25436
Winterbourn CC, Hampton MB (2008) Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45:549–561. https://doi.org/10.1016/j.freeradbiomed.2008.05.004
Wirthgen E, Hoeflich A (2015) Endotoxin-induced tryptophan degradation along the kynurenine pathway: the role of Indolamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immunosuppressive effects in endotoxin tolerance and Cancer and its implications for Immunoparalysis. J Amino Acids 2015:973548. https://doi.org/10.1155/2015/973548
Wisnik E, Pikus E, Duchnowicz P, Koter-Michalak M (2017) Tolerance of monocytes and macrophages in response to bacterial endotoxin. Postepy higieny i medycyny doswiadczalnej 71:176–185
Wong N, Nguyen T, Brenu EW, Broadley S, Staines D, Marshall-Gradisnik S (2015) A comparison of cytokine profiles of chronic fatigue syndrome/Myalgic encephalomyelitis and multiple sclerosis patients international. J Clin Med 06:769–783. https://doi.org/10.4236/ijcm.2015.610103
Wortinger LA, Endestad T, Melinder AM, Oie MG, Sevenius A, Bruun Wyller V (2016) Aberrant resting-state functional connectivity in the salience network of adolescent chronic fatigue syndrome. PLoS One 11:e0159351. https://doi.org/10.1371/journal.pone.0159351
Wu C et al (2010) Redox regulatory mechanism of transnitrosylation by thioredoxin. Mol Cell Proteomics 9:2262–2275. https://doi.org/10.1074/mcp.M110.000034
Wu C et al (2011) Thioredoxin 1-mediated post-translational modifications: reduction, transnitrosylation, denitrosylation, and related proteomics methodologies. Antioxid Redox Signal 15:2565–2604. https://doi.org/10.1089/ars.2010.3831
Wu Y, Sun Q, Dai L (2017) Immune regulation of miR-30 on the mycobacterium tuberculosis-induced TLR/MyD88 signaling pathway in THP-1 cells. Exp Ther Med 14:3299–3303. https://doi.org/10.3892/etm.2017.4872
Wyller VB, Nguyen CB, Ludviksen JA, Mollnes TE (2017) Transforming growth factor beta (TGF-beta) in adolescent chronic fatigue syndrome. J Transl Med 15:245. https://doi.org/10.1186/s12967-017-1350-1
Xavier AL, Menezes JRL, Goldman SA, Nedergaard M (2014) Fine-tuning the central nervous system: microglial modelling of cells and synapses. Philos Trans R Soc Lond B Biol Sci 369:20130593. https://doi.org/10.1098/rstb.2013.0593
Yamano E et al (2016) Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci Rep 6:34990. https://doi.org/10.1038/srep34990
Yan Z, Lira VA, Greene NP (2012) Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev 40:159–164. https://doi.org/10.1097/JES.0b013e3182575599
Yang H et al (2012) SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS One 7:e46364. https://doi.org/10.1371/journal.pone.0046364
Yang Y, Sun H, Li X, Ding Q, Wei P, Zhou J (2015) Transforming growth factor Beta-induced is essential for endotoxin tolerance induced by a low dose of lipopolysaccharide in human peripheral blood mononuclear cells. Iran J Allergy Asthma Immunol 14:321–330
Yasinska IM, Sumbayev VV (2003) S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett 549:105–109
Ye X, Hemida MG, Qiu Y, Hanson PJ, Zhang HM, Yang D (2013) MiR-126 promotes coxsackievirus replication by mediating cross-talk of ERK1/2 and Wnt/beta-catenin signal pathways. Cell Mol Life Sci 70:4631–4644. https://doi.org/10.1007/s00018-013-1411-4
Yi JS, Cox MA, Zajac AJ (2010) T-cell exhaustion: characteristics, causes and conversion. Immunology 129:474–481. https://doi.org/10.1111/j.1365-2567.2010.03255.x
Yoshiuchi K, Farkas J, Natelson B (2006) Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging 26:83–86
Yoza BK, McCall CE (2011) Facultative heterochromatin formation at the IL-1 beta promoter in LPS tolerance and sepsis. Cytokine 53:145–152. https://doi.org/10.1016/j.cyto.2010.10.007
Yoza BK, Hu JYQ, Cousart SL, Forrest LM, McCall CE (2006) Induction of RelB participates in endotoxin tolerance. J Immunol 177:4080–4085. https://doi.org/10.4049/jimmunol.177.6.4080
Yu Q, Xiang L, Yin L, Liu X, Yang D, Zhou J (2017) Loss-of-function of miR-142 by hypermethylation promotes TGF-beta-mediated tumour growth and metastasis in hepatocellular carcinoma. Cell Prolif 50. https://doi.org/10.1111/cpr.12384
Zago M, Rico de Souza A, Hecht E, Rousseau S, Hamid Q, Eidelman DH, Baglole CJ (2014) The NF-kappaB family member RelB regulates microRNA miR-146a to suppress cigarette smoke-induced COX-2 protein expression in lung fibroblasts. Toxicol Lett 226:107–116. https://doi.org/10.1016/j.toxlet.2014.01.020
Zago M et al (2017) Low levels of the AhR in chronic obstructive pulmonary disease (COPD)-derived lung cells increases COX-2 protein by altering mRNA stability. PLoS One 12:e0180881. https://doi.org/10.1371/journal.pone.0180881
Zhang Y, Zhang H (2013) Microbiota associated with type 2 diabetes and its related complications. Food Sci Human Wellness 2:167–172. https://doi.org/10.1016/j.fshw.2013.09.002
Zhang J, Jin B, Li L, Block E, Patel J (2005) Nitric oxide-induced persistent inhibition and nitrosylation of active site cysteine residues of mitochondrial cytochrome-c oxidase in lung endothelial cells. Am J Phys Cell Phys 288:C840–C849
Zhang L et al (2010) Microbial infections in eight genomic subtypes of chronic fatigue syndrome/myalgic encephalomyelitis. J Clin Pathol 63:156–164. https://doi.org/10.1136/jcp.2009.072561
Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Shannon Weickert C (2016) Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry 6:e982. https://doi.org/10.1038/tp.2016.238 https://www.nature.com/articles/tp2016238#supplementary-information
Zheng L, Leung E, Lee N, Lui G, To KF, Chan RC, Ip M (2015) Differential MicroRNA expression in human macrophages with mycobacterium tuberculosis infection of Beijing/W and non-Beijing/W strain types. PLoS One 10:e0126018. https://doi.org/10.1371/journal.pone.0126018
Zhong L et al (2010) The histone deacetylase SIRT6 regulates glucose homeostasis via Hif1α. Cell 140:280. https://doi.org/10.1016/j.cell.2009.12.041
Zhou QF, Xu SM, Wang HQ, Xing LM, Fu R, Shao ZH (2017) Single nucleotide polymorphism of mitochondrial DNA D-LOOP region in peripheral blood lymphocytes of immuno-related pancytopenia patients. Zhongguo shi yan xue ye xue za zhi 25:186–191. https://doi.org/10.7534/j.issn.1009-2137.2017.01.033
Acknowledgments
MB is supported by a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (grant number 1059660).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Morris, G., Maes, M., Berk, M. et al. Myalgic encephalomyelitis or chronic fatigue syndrome: how could the illness develop?. Metab Brain Dis 34, 385–415 (2019). https://doi.org/10.1007/s11011-019-0388-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-0388-6