Abstract
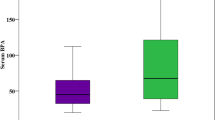
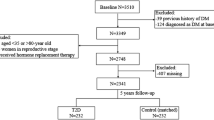
There is a striking interaction of genes and environment in the etiology of type 2 diabetes mellitus (T2DM). While endocrine disrupting chemicals (EDCs) like bisphenol-A (BPA) have received special attention for their mechanistic role in metabolic disruption, there is a lack of clinically relevant data on BPA levels in Asian Indians, a population which is more susceptible to type 2 diabetes mellitus (T2DM) and cardiovascular diseases. Therefore, we measured systemic levels of BPA in patients with T2DM compared to individuals with normal glucose tolerance (n = 30 each). Serum BPA levels were estimated using ELISA kit, and biochemical determinations were done by standard protocols. Peripheral blood mononuclear cells (PBMCs) were used to profile the gene expression alterations with special reference to inflammation, estrogen receptors, and cellular senescence in these subjects. Serum levels of BPA were significantly higher in patients with T2DM compared to control individuals and positively correlated to poor glycemic control and insulin resistance. Patients with T2DM exhibited significantly elevated mRNA levels of senescence (GLB1, p16, p21, and p53) and inflammatory (IL6 and TNF-α) markers, shortened telomeres as well as elevated levels of estrogen-related receptor gamma (ERRγ), a recently identified receptor for BPA. BPA levels were positively correlated to senescence indicators, inflammatory markers and ERRγ and negatively correlated to telomere length. Our study is the first data in the clinical diabetes setting to demonstrate an association of increased BPA levels with cellular senescence, proinflammation, poor glycemic control, insulin resistance, and shortened telomeres in patients with T2DM.
Graphical abstract







Similar content being viewed by others
References
Jaacks LM, Siegel KR, Gujral UP, Narayan KM (2016) Type 2 diabetes: a 21st century epidemic. Best Pract Res Clin Endocrinol Metab 30:331–343. https://doi.org/10.1016/j.beem.2016.05.003
International Diabetes Federation (2017) IDF diabetes atlas, 8th edn. International Diabetes Federation, Brussels
Kaul N, Ali S (2016) Genes, genetics, and environment in type 2 diabetes: implication in personalized medicine. DNA Cell Biol 35:1–12. https://doi.org/10.1089/dna.2015.2883
Defronzo RA (2009) Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795. https://doi.org/10.2337/db09-9028
Imamura M, Takahashi A, Yamauchi T, Hara K, Yasuda K, Grarup N, Zhao W, Wang X, Huerta-Chagoya A, Hu C, Moon S, Long J, Kwak SH, Rasheed A, Saxena R, Ma RC, Okada Y, Iwata M, Hosoe J, Shojima N, Iwasaki M, Fujita H, Suzuki K, Danesh J, Jorgensen T, Jorgensen ME, Witte DR, Brandslund I, Christensen C, Hansen T, Mercader JM, Flannick J, Moreno-Macias H, Burtt NP, Zhang R, Kim YJ, Zheng W, Singh JR, Tam CH, Hirose H, Maegawa H, Ito C, Kaku K, Watada H, Tanaka Y, Tobe K, Kawamori R, Kubo M, Cho YS, Chan JC, Sanghera D, Frossard P, Park KS, Shu XO, Kim BJ, Florez JC, Tusie-Luna T, Jia W, Tai ES, Pedersen O, Saleheen D, Maeda S, Kadowaki T (2016) Genome-wide association studies in the Japanese population identify seven novel loci for type 2 diabetes. Nat Commun 7:10531. https://doi.org/10.1038/ncomms10531
Chevalier N, Fenichel P (2016) Endocrine disruptors: a missing link in the pandemy of type 2 diabetes and obesity? Presse Med 45:88–97. https://doi.org/10.1016/j.lpm.2015.08.008
Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F (2017) Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 68:3–33. https://doi.org/10.1016/j.reprotox.2016.10.001
Mimoto MS, Nadal A, Sargis RM (2017) Polluted pathways: mechanisms of metabolic disruption by endocrine disrupting chemicals. Curr Environ Health Rep 4:208–222. https://doi.org/10.1007/s40572-017-0137-0
Fenichel P, Chevalier N, Brucker-Davis F (2013) Bisphenol A: an endocrine and metabolic disruptor. Ann Endocrinol (Paris) 74:211–220. https://doi.org/10.1016/j.ando.2013.04.002
Provvisiero DP, Pivonello C, Muscogiuri G, Negri M, de Angelis C, Simeoli C, Pivonello R, Colao A (2016) Influence of bisphenol A on type 2 diabetes mellitus. Int J Environ Res Public Health 13:989. https://doi.org/10.3390/ijerph13100989
Sowlat MH, Lotfi S, Yunesian M, Ahmadkhaniha R, Rastkari N (2016) The association between bisphenol A exposure and type-2 diabetes: a world systematic review. Environ Sci Pollut Res Int 23:21125–21140. https://doi.org/10.1007/s11356-016-7525-0
Chailurkit LO, Tengpraettanakorn P, Chanprasertyotin S, Ongphiphadhanakul B (2017) Is bisphenol A exposure associated with the development of glucose intolerance and increased insulin resistance in Thais? Nutr Health 23:185–191. https://doi.org/10.1177/0260106017708730
Gujral UP, Narayan KM, Pradeepa RG, Deepa M, Ali MK, Anjana RM, Kandula NR, Mohan V, Kanaya AM (2015) Comparing type 2 diabetes, prediabetes, and their associated risk factors in Asian Indians in India and in the U.S.: the CARRS and MASALA studies. Diabetes Care 38:1312–1318. https://doi.org/10.2337/dc15-0032
Shah VN, Mohan V (2015) Diabetes in India: what is different? Curr Opin Endocrinol Diabetes Obes 22:283–289. https://doi.org/10.1097/MED.0000000000000166
Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL (2015) Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 64:2289–2298. https://doi.org/10.2337/db14-1820
Regulski M (2018) Understanding diabetic induction of cellular senescence: a concise review. Wounds 30:96–101
Adaikalakoteswari A, Balasubramanyam M, Mohan V (2005) Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabet Med 22:1151–1156. https://doi.org/10.1111/j.1464-5491.2005.01574.x
Qin XY, Fukuda T, Yang L, Zaha H, Akanuma H, Zeng Q, Yoshinaga J, Sone H (2012) Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer Biol Ther 13:296–306. https://doi.org/10.4161/cbt.18942
Herz C, Tran HTT, Schlotz N, Michels K, Lamy E (2017) Low-dose levels of bisphenol A inhibit telomerase via ER/GPR30-ERK signalling, impair DNA integrity and reduce cell proliferation in primary PBMC. Sci Rep 7:16631. https://doi.org/10.1038/s41598-017-15978-2
Katchy A, Pinto C, Jonsson P, Nguyen-Vu T, Pandelova M, Riu A, Schramm KW, Samarov D, Gustafsson JA, Bondesson M, Williams C (2014) Coexposure to phytoestrogens and bisphenol a mimics estrogenic effects in an additive manner. Toxicol Sci 138:21–35. https://doi.org/10.1093/toxsci/kft271
Bansal A, Henao-Mejia J, Simmons RA (2018) Immune system: an emerging player in mediating effects of endocrine disruptors on metabolic health. Endocrinology 159:32–45. https://doi.org/10.1210/en.2017-00882
Elfaki I, Mir R, Almutairi FM, Duhier FMA (2018) Cytochrome P450: polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac J Cancer Prev 19:2057–2070. https://doi.org/10.22034/APJCP.2018.19.8.2057
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Triant DA, Whitehead A (2009) Simultaneous extraction of high-quality RNA and DNA from small tissue samples. J Hered 100:246–250. https://doi.org/10.1093/jhered/esn083
Monickaraj F, Gokulakrishnan K, Prabu P, Sathishkumar C, Anjana RM, Rajkumar JS, Mohan V, Balasubramanyam M (2012) Convergence of adipocyte hypertrophy, telomere shortening and hypoadiponectinemia in obese subjects and in patients with type 2 diabetes. Clin Biochem 45:1432–1438. https://doi.org/10.1016/j.clinbiochem.2012.07.097
Wiley CD, Campisi J (2016) From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab 23:1013–1021. https://doi.org/10.1016/j.cmet.2016.05.010
Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, Morisset AS, Taback S, Bouchard MF, Monnier P, Dallaire R, Fraser WD (2014) A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environ Health 13:84. https://doi.org/10.1186/1476-069X-13-84
Garcia-Arevalo M, Alonso-Magdalena P, Rebelo Dos Santos J, Quesada I, Carneiro EM, Nadal A (2014) Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS ONE 9:e100214. https://doi.org/10.1371/journal.pone.0100214
Shankar A, Teppala S (2011) Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab 96:3822–3826. https://doi.org/10.1210/jc.2011-1682
Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB (2014) Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect 122:616–623. https://doi.org/10.1289/ehp.1307201
Aekplakorn W, Chailurkit LO, Ongphiphadhanakul B (2015) Relationship of serum bisphenol A with diabetes in the Thai population, National Health Examination Survey IV, 2009. J Diabetes 7:240–249. https://doi.org/10.1111/1753-0407.12159
Andra SS, Kalyvas H, Andrianou XD, Charisiadis P, Christophi CA, Makris KC (2015) Preliminary evidence of the association between monochlorinated bisphenol A exposure and type II diabetes mellitus: a pilot study. J Environ Sci Health A 50:243–259. https://doi.org/10.1080/10934529.2015.981111
Kirkland JL, Tchkonia T (2017) Cellular senescence: a translational perspective. EBioMedicine 21:21–28. https://doi.org/10.1016/j.ebiom.2017.04.013
Shiny A, Regin B, Balachandar V, Gokulakrishnan K, Mohan V, Babu S, Balasubramanyam M (2013) Convergence of innate immunity and insulin resistance as evidenced by increased nucleotide oligomerization domain (NOD) expression and signaling in monocytes from patients with type 2 diabetes. Cytokine 64:564–570. https://doi.org/10.1016/j.cyto.2013.08.003
Negroni A, Pierdomenico M, Cucchiara S, Stronati L (2018) NOD2 and inflammation: current insights. J Inflamm Res 11:49–60. https://doi.org/10.2147/JIR.S137606
Ribeiro-Varandas E, Pereira HS, Monteiro S, Neves E, Brito L, Ferreira RB, Viegas W, Delgado M (2014) Bisphenol A disrupts transcription and decreases viability in aging vascular endothelial cells. Int J Mol Sci 15:15791–15805. https://doi.org/10.3390/ijms150915791
Jalal N, Surendranath AR, Pathak JL, Yu S, Chung CY (2018) Bisphenol A (BPA) the mighty and the mutagenic. Toxicol Rep 5:76–84. https://doi.org/10.1016/j.toxrep.2017.12.013
Alonso-Magdalena P, Ropero AB, Soriano S, Garcia-Arevalo M, Ripoll C, Fuentes E, Quesada I, Nadal A (2012) Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol 355:201–207. https://doi.org/10.1016/j.mce.2011.12.012
Tohme M, Prud’homme SM, Boulahtouf A, Samarut E, Brunet F, Bernard L, Bourguet W, Gibert Y, Balaguer P, Laudet V (2014) Estrogen-related receptor gamma is an in vivo receptor of bisphenol A. FASEB J 28:3124–3133. https://doi.org/10.1096/fj.13-240465
Misra J, Kim DK, Jung YS, Kim HB, Kim YH, Yoo EK, Kim BG, Kim S, Lee IK, Harris RA, Kim JS, Lee CH, Cho JW, Choi HS (2016) O-glcnacylation of orphan nuclear receptor estrogen-related receptor gamma promotes hepatic gluconeogenesis. Diabetes 65:2835–2848. https://doi.org/10.2337/db15-1523
Misra J, Kim DK, Choi HS (2017) ERRγ: a junior orphan with a senior role in metabolism. Trends Endocrinol Metab 28:261–272. https://doi.org/10.1016/j.tem.2016.12.005
Huang Q, Chen Q (2017) Mediating roles of PPARs in the effects of environmental chemicals on sex steroids. PPAR Res 2017:3203161. https://doi.org/10.1155/2017/3203161
Gan Q, Huang J, Zhou R, Niu J, Zhu X, Wang J, Zhang Z, Tong T (2008) PPARγ accelerates cellular senescence by inducing p16INK4α expression in human diploid fibroblasts. J Cell Sci 121:2235–2245. https://doi.org/10.1242/jcs.026633
Goldstein JA, de Morais SM (1994) Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics 4:285–299
Xu JY, Wu L, Shi Z, Zhang XJ, Englert NA, Zhang SY (2017) Upregulation of human CYP2C9 expression by Bisphenol A via estrogen receptor alpha (ERalpha) and Med25. Environ Toxicol 32:970–978. https://doi.org/10.1002/tox.22297
Holstein A, Plaschke A, Ptak M, Egberts EH, El-Din J, Brockmoller J, Kirchheiner J (2005) Association between CYP2C9 slow metabolizer genotypes and severe hypoglycaemia on medication with sulphonylurea hypoglycaemic agents. Br J Clin Pharmacol 60:103–106. https://doi.org/10.1111/j.1365-2125.2005.02379.x
Castelan-Martinez OD, Hoyo-Vadillo C, Bazan-Soto TB, Cruz M, Tesoro-Cruz E, Valladares-Salgado A (2018) CYP2C9*3 gene variant contributes independently to glycaemic control in patients with type 2 diabetes treated with glibenclamide. J Clin Pharm Ther. https://doi.org/10.1111/jcpt.12710
Rao DK, Murthy DK, Shaik NS, Banaganapalli B, Konda K, Rao HP, Ganti E, Ahmed Awan Z, A El‐Harouni A, Elango R, Ali Khan I, Shaik NA (2017) Distribution of CYP2C8 and CYP2C9 amino acid substitution alleles in South Indian diabetes patients: a genotypic and computational protein phenotype study. Clin Exp Pharmacol Physiol 44:1171–1179. https://doi.org/10.1111/1440-1681.12810
Rabiee M, Marjani A, Khajeniazi S, Mojerloo M (2018) Genetic polymorphisms of cytochrome p450 (2C9) enzyme in patients with type 2 diabetes mellitus in Turkmen and Fars Ethnic Groups. Endocr Metab Immune Disord Drug Targets 18:653–661. https://doi.org/10.2174/1871530318666180821122853
Zhou Y, Simmons D, Hambly BD, McLachlan CS (2016) Interactions between UCP2 SNPs and telomere length exist in the absence of diabetes or pre-diabetes. Sci Rep 6:33147. https://doi.org/10.1038/srep33147
Zhang X, Lin S, Funk WE, Hou L (2013) Environmental and occupational exposure to chemicals and telomere length in human studies. Occup Environ Med 70:743–749. https://doi.org/10.1136/oemed-2012-101350
Ziegler S, Schettgen T, Beier F, Wilop S, Quinete N, Esser A, Masouleh BK, Ferreira MS, Vankann L, Uciechowski P, Rink L, Kraus T, Brummendorf TH, Ziegler P (2017) Accelerated telomere shortening in peripheral blood lymphocytes after occupational polychlorinated biphenyls exposure. Arch Toxicol 91:289–300. https://doi.org/10.1007/s00204-016-1725-8
Guzzardi MA, Iozzo P, Salonen MK, Kajantie E, Airaksinen R, Kiviranta H, Rantakokko P, Eriksson JG (2016) Exposure to persistent organic pollutants predicts telomere length in older age: results from the Helsinki Birth Cohort Study. Aging Dis 7:540–552. https://doi.org/10.14336/AD.2016.0209
Yuan J, Liu Y, Wang J, Zhao Y, Li K, Jing Y, Zhang X, Liu Q, Geng X, Li G, Wang F (2018) Long-term persistent organic pollutants exposure induced telomere dysfunction and senescence-associated secretary phenotype. J Gerontol A. https://doi.org/10.1093/gerona/gly002
Caporossi L, Papaleo B (2015) Exposure to bisphenol A and gender differences: from rodents to humans evidences and hypothesis about the health effects. J Xenobiotics 5(1):5. https://doi.org/10.4081/xeno.2015.5264
Awada Z, Sleiman F, Mailhac A, Mouneimne Y, Tamim H, Zgheib NK (2018) BPA exposure is associated with non-monotonic alteration in ESR1 promoter methylation in peripheral blood of men and shorter relative telomere length in peripheral blood of women. J Expo Sci Environ Epidemiol. https://doi.org/10.1038/s41370-018-0030-4
Bhavadharini B, Mahalakshmi MM, Anjana RM, Maheswari K, Uma R, Deepa M, Unnikrishnan R, Ranjani H, Pastakia SD, Kayal A, Ninov L, Malanda B, Belton A, Mohan V (2016) Prevalence of Gestational Diabetes Mellitus in urban and rural Tamil Nadu using IADPSG and WHO 1999 criteria (WINGS 6). Clin Diabetes Endocrinol 2:8. https://doi.org/10.1186/s40842-016-0028-6
Ehrlich S, Lambers D, Baccarelli A, Khoury J, Macaluso M, Ho SM (2016) Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. Am J Perinatol 33:1313–1318. https://doi.org/10.1055/s-0036-1586500
Tan L, Wang S, Wang Y, He M, Liu D (2015) Bisphenol A exposure accelerated the aging process in the nematode Caenorhabditis elegans. Toxicol Lett 235:75–83. https://doi.org/10.1016/j.toxlet.2015.03.010
Mahemuti L, Chen Q, Coughlan MC, Qiao C, Chepelev NL, Florian M, Dong D, Woodworth RG, Yan J, Cao XL, Scoggan KA, Jin X, Willmore WG (2018) Bisphenol A induces DSB-ATM-p53 signaling leading to cell cycle arrest, senescence, autophagy, stress response, and estrogen release in human fetal lung fibroblasts. Arch Toxicol 92:1453–1469. https://doi.org/10.1007/s00204-017-2150-3
WHO (World Health Organization) (2015) Strategic Approach to International Chemicals Management: implementation and priorities in the health sector. Report from the WHO Regional Office for Europe, Bonn, pp 1–31
Stahlhut RW, Myers JP, Taylor JA, Nadal A, Dyer JA, Vom Saal FS (2018) Experimental BPA exposure and glucose-stimulated insulin response in adult men and women. J Endocr Soc 2:1173–1187. https://doi.org/10.1210/js.2018-00151
Acknowledgements
Authors thankfully acknowledge the seed money grant from MDRF Intramural Research Funding (MIRF). Authors also acknowledge the partial financial assistance from the Department of Biotechnology (DBT), Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no actual or potential competing financial interests. Authors also declare that they do not have any non-financial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soundararajan, A., Prabu, P., Mohan, V. et al. Novel insights of elevated systemic levels of bisphenol-A (BPA) linked to poor glycemic control, accelerated cellular senescence and insulin resistance in patients with type 2 diabetes. Mol Cell Biochem 458, 171–183 (2019). https://doi.org/10.1007/s11010-019-03540-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03540-9