Abstract
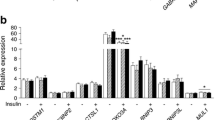
Diabetes mellitus (DM) induces a variable degree of muscle sarcopenia, which may be related to protein degradation and to the expression of both E3 ubiquitin ligases and some specific microRNAs (miRNAs). The present study investigated the effect of diabetes and acute muscle contraction upon the TRIM63 and FBXO32 expression as well as the potential involvement of some miRNAs. Diabetes was induced by streptozotocin and studied after 30 days. Soleus muscles were harvested, stimulated to contract in vitro for twitch tension analysis (0.5 Hz), 30 min later for tetanic analysis (100 Hz), and 30 min later were frozen. TRIM63 and FBXO32 proteins were quantified by western blotting; Trim63 mRNA, Fbxo32 mRNA, miR-1-3p, miR-29a-3p, miR-29b-3p, miR-133a-3p, and miR-133b-3p were quantified by qPCR. Diabetes induced sarcopenia by decreasing (P < 0.05) muscle weight/tibia length index, maximum tetanic contraction and relaxation rates, and absolute twitch and tetanic forces (P < 0.05). Diabetes decreased (P < 0.05) the Trim63 and Fbxo32 mRNAs (30%) and respective proteins (60%), and increased (P < 0.01) the miR-29b-3p (2.5-fold). In muscle from diabetic rats, acute contractile stimulus increased TRIM63 protein, miR-1-3p, miR-29a-3p, and miR-133a/b-3p, but decreased miR-29b-3p (P < 0.05). Independent of the metabolic condition, after muscle contraction, both TRIM63 and FBXO32 proteins correlated significantly with miR-1-3p, miR-29a/b-3p, and miR-133a/b-3p. All diabetes-induced regulations were reversed by insulin treatment. Concluding, the results depict that muscle wasting in long-term insulinopenic condition may not be accompanied by increased proteolysis, pointing out the protein synthesis as an important modulator of muscle sarcopenia in DM.





Similar content being viewed by others
References
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14
Thomas CC, Philipson LH (2015) Update on diabetes classification. Med Clin N Am 99:1–16
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Sun Z, Liu L, Liu N, Liu Y (2008) Muscular response and adaptation to diabetes mellitus. Front Biosci 13:4765–4794
Marin P, Andersson B, Krotkiewski M, Bjorntorp P (1994) Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17:382–386
Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Blüher M, Punkt K (2006) Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29:895–900
Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Jarvinen H, Christin L, Secomb TW, Bogardus C (1987) Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Investig 80:415–424
Hudlicka O (1985) Development and adaptability of microvasculature in skeletal muscle. J Exp Biol 115:215–228
Wang Y, Pessin JE (2013) Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 16:243–250
Gordon BS, Kelleher AR, Kimball SR (2013) Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol 45:2147–2157
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18:39–51
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Chen H, Lan HY, Roukos DH, Cho WC (2014) Application of microRNAs in diabetes mellitus. J Endocrinol 222:R1–R10
Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C (2007) Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci 4:19–27
Bassil MS, Gougeon R (2013) Muscle protein anabolism in type 2 diabetes. Curr Opin Clin Nutr Metab Care 16:83–88
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273:E99–E107
Fedele MJ, Hernandez JM, Lang CH, Vary TC, Kimball SR, Jefferson LS, Farrell PA (2000) Severe diabetes prohibits elevations in muscle protein synthesis after acute resistance exercise in rats. J Appl Physiol 88:102–108
Lima GA, Anhe GF, Giannocco G, Nunes MT, Correa-Giannella ML, Machado UF (2009) Contractile activity per se induces transcriptional activation of SLC2A4 gene in soleus muscle: involvement of MEF2D, HIF-1a, and TRalpha transcriptional factors. Am J Physiol Endocrinol Metab 296:E132–E138
Granjon A, Gustin MP, Rieusset J, Lefai E, Meugnier E, Guller I, Cerutti C, Paultre C, Disse E, Rabasa-Lhoret R, Laville M, Vidal H, Rome S (2009) The microRNA signature in response to insulin reveals its implication in the transcriptional action of insulin in human skeletal muscle and the role of a sterol regulatory element-binding protein-1c/myocyte enhancer factor 2C pathway. Diabetes 58:2555–2564
Chen GQ, Mou CY, Yang YQ, Wang S, Zhao ZW (2011) Exercise training has beneficial anti-atrophy effects by inhibiting oxidative stress-induced MuRF1 upregulation in rats with diabetes. Life Sci 89:44–49
Russell AP, Lamon S, Boon H, Wada S, Guller I, Brown EL, Chibalin AV, Zierath JR, Snow RJ, Stepto N, Wadley GD, Akimoto T (2013) Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training. J Physiol 591:4637–4653
Okamoto MM, Sumida DH, Carvalho CR, Vargas AM, Heimann JC, Schaan BD, Machado UF (2004) Changes in dietary sodium consumption modulate GLUT4 gene expression and early steps of insulin signaling. Am J Physiol Regul Integr Comp Physiol 286:R779–R785
Henriksen EJ, Rodnick KJ, Mondon CE, James DE, Holloszy JO (1991) Effect of denervation or unweighting on GLUT-4 protein in rat soleus muscle. J Appl Physiol 70:2322–2327
Silva JL, Giannocco G, Furuya DT, Lima GA, Moraes PA, Nachef S, Bordin S, Britto LR, Nunes MT, Machado UF (2005) NF-kappaB, MEF2A, MEF2D and HIF1-a involvement on insulin- and contraction-induced regulation of GLUT4 gene expression in soleus muscle. Mol Cell Endocrinol 240:82–93
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Dweep H, Gretz N (2015) miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat Methods 12:697
Andersen H, Poulsen PL, Mogensen CE, Jakobsen J (1996) Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes 45:440–445
Punkt K, Psinia I, Welt K, Barth W, Asmussen G (1999) Effects on skeletal muscle fibres of diabetes and Ginkgo biloba extract treatment. Acta Histochem 101:53–69
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103:16260–16265
Dehoux M, Van Beneden R, Pasko N, Lause P, Verniers J, Underwood L, Ketelslegers JM, Thissen JP (2004) Role of the insulin-like growth factor I decline in the induction of atrogin-1/MAFbx during fasting and diabetes. Endocrinology 145:4806–4812
Chen Y, Cao L, Ye J, Zhu D (2009) Upregulation of myostatin gene expression in streptozotocin-induced type 1 diabetes mice is attenuated by insulin. Biochem Biophys Res Commun 388:112–116
Paula-Gomes S, Goncalves DA, Baviera AM, Zanon NM, Navegantes LC, Kettelhut IC (2013) Insulin suppresses atrophy- and autophagy-related genes in heart tissue and cardiomyocytes through AKT/FOXO signaling. Horm Metab Res 45:849–855
Vignaud A, Ramond F, Hourde C, Keller A, Butler-Browne G, Ferry A (2007) Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology 74:291–300
Lambertucci AC, Lambertucci RH, Hirabara SM, Curi R, Moriscot AS, Alba-Loureiro TC, Guimaraes-Ferreira L, Levada-Pires AC, Vasconcelos DA, Sellitti DF, Pithon-Curi TC (2012) Glutamine supplementation stimulates protein-synthetic and inhibits protein-degradative signaling pathways in skeletal muscle of diabetic rats. PLoS ONE 7(12):e50390
Fagan JM, Satarug S, Cook P, Tischler ME (1987) Rat muscle protein turnover and redox state in progressive diabetes. Life Sci 40:783–790
Galban VD, Evangelista EA, Migliorini RH, do Carmo Kettelhut I (2001) Role of ubiquitin-proteasome-dependent proteolytic process in degradation of muscle protein from diabetic rabbits. Mol Cell Biochem 225:35–41
Foletta VC, White LJ, Larsen AE, Leger B, Russell AP (2011) The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflug Arch 461:325–335
Kim HJ, Lee JS, Kim CK (2004) Effect of exercise training on muscle glucose transporter 4 protein and intramuscular lipid content in elderly men with impaired glucose tolerance. Eur J Appl Physiol 93:353–358
Lecker SH, Solomon V, Price SR, Kwon YT, Mitch WE, Goldberg AL (1999) Ubiquitin conjugation by the N-end rule pathway and mRNAs for its components increase in muscles of diabetic rats. J Clin Investig 104:1411–1420
Soares RJ, Cagnin S, Chemello F, Silvestrin M, Musaro A, De Pitta C, Lanfranchi G, Sandri M (2014) Involvement of microRNAs in the regulation of muscle wasting during catabolic conditions. J Biol Chem 289:21909–21925
He A, Zhu L, Gupta N, Chang Y, Fang F (2007) Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol 21:2785–2794
Melnik BC (2015) The pathogenic role of persistent milk signaling in mTORC1- and milk-microRNA-driven type 2 diabetes mellitus. Curr Diabetes Rev 11:46–62
McCarthy JJ, Esser KA (2007) MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol 102:306–313
O’Neill ED, Wilding JP, Kahn CR, Van Remmen H, McArdle A, Jackson MJ, Close GL (2010) Absence of insulin signalling in skeletal muscle is associated with reduced muscle mass and function: evidence for decreased protein synthesis and not increased degradation. Age (Dordr) 32:209–222
Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL (2007) Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21:140–155
Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, LaStayo PC, Marcus RL (2014) Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: a cross-sectional comparison. J Gerontol A 69:1040–1048
Acknowledgements
This research was supported by the National Council for Scientific and Technological Development (CNPq) and by the São Paulo Research Foundation (FAPESP) #2012/04831-1. The authors are grateful to Dr. Antonio Carlos Oliveira and Ms. Carmen S. M. Serra for technical assistance and to Dr. Adauri Brezolin for English revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gerlinger-Romero, F., Yonamine, C.Y., Junior, D.C.P. et al. Dysregulation between TRIM63/FBXO32 expression and soleus muscle wasting in diabetic rats: potential role of miR-1-3p, -29a/b-3p, and -133a/b-3p. Mol Cell Biochem 427, 187–199 (2017). https://doi.org/10.1007/s11010-016-2910-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2910-z