Summary
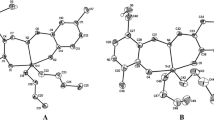
Dibutyltin(IV) complexes of composition Bu2Sn(LH)2, where LH is a carboxylate residue derived from 2-[(E)-(5-tert-butyl-2-hydroxyphenyl)diazenyl]benzoate (L1H) with water molecule (1), 4-[(E)-(5-tert-butyl-2-hydroxyphenyl)diazenyl]benzoate (L2H) (2) and 4-[(E)-(4-hydroxy-5-methylphenyl)diazenyl]benzoate (L3H) (3), were synthesized and characterized by spectroscopic (1H, 13C and 119Sn NMR, IR, 119Sn Mössbauer) techniques. A full characterization was accomplished from the crystal structure of complex 1. The molecular structures and geometries of the complexes (1a i.e. 1 without water molecule and 3) were fully optimized using the quantum mechanical method (PM6). Complexes 1 and 3 were found to exhibit stronger cytotoxic activity in vitro across a panel of human tumor cell lines viz., A498, EVSA-T, H226, IGROV, M19 MEL, MCF-7 and WIDR. Compound 3 is found to be four times superior for the A498, EVSA-T and MCF-7 cell lines than CCDP (cisplatin), and four, eight and sixteen times superior for the A498, H226 and MCF-7 cell lines, respectively, compared to ETO (etoposide). The mechanistic role of cytotoxic activity of test compounds is discussed in relation to the theoretical results of docking studies with some key enzymes such as ribonucleotide reductase, thymidylate synthase, thymidylate phosphorylase and topoisomerase II associated with the propagation of cancer.











Similar content being viewed by others
References
Abu-Surrah AS, Kettunen M (2006) Platinum group antitumor chemistry: design and development of new anticancer drugs complementary to cisplatin. Curr Med Chem 13:1337–1357
Barnes KR, Lippard SJ (2004) Metal complexes in tumor diagnosis and as anticancer agents. Met Ions Biol Syst 42:143–177
Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM (2007) Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem 7:3–18
Yang P, Guo M (1999) Interactions of organometallic anticancer agents with nucleotides and DNA. Coord Chem Rev 185–186:189–211
Reedijk J (2003) New clues for platinum antitumor chemistry: kinetically controlled metal binding to DNA. Proc Natl Acad Sci USA 100:3611–3616
Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer. Nat Rev Drug Discov 4:307–320
Sigel A, Sigel H (2004) Metal ions in biological systems. Marcel Dekkar, New York
Jakupec M, Keppler BK (2004) In: Sigel A, Sigel H (eds) Metal ions in biological systems. Marcel Dekker, New York
Alessio E, Mestroni G, Bergamo A, Sava G (2004) Ruthenium antimetastatic agents. Curr Top Med Chem 4:1525–1535
Sigel A, Sigel H (2005) Metal ions in biological systems. Wiley, New York
Gielen M, Tiekink ERT (eds) (2005) Metallotherapeutic drug and metal-based diagnostic agents. Wiley, Chichester, England
Ang WH, Dyson PJ (2006) Classical and non-classical ruthenium-based anticancer drugs: towards targeted chemotherapy (review). Eur J Inorg Chem 4003–4018
Bruijnincx PCA, Sadler PJ (2008) New trends for metal complexes with anticancer activity. Curr Opin Chem Biol 12:197–206
Tiekink ERT (2008) Anti-cancer potential of gold complexes. Inflammopharmacol 16:138–142
Hadjikakou SK, Hadjiliadis N (2009) Antiproliferative and anti-tumor activity of organotin compounds. Coord Chem Rev 253:235–249
Tiekink ERT (1994) The rich diversity in tin carboxylate structures. Trends Organomet Chem 1:71–116
Tiekink ERT (1991) Structural chemistry of organotin carboxylates: a review of the crystallographic literature. Appl Organomet Chem 5:1–21
Chandrasekhar V, Nagendran S, Baskar V (2002) Organotin assemblies containing Sn–-O bonds. Coord Chem Rev 235:1–52
Buntine MA, Hall VJ, Kosovel FJ, Tiekink ERT (1998) Influence of crystal packing on molecular geometry: a crystallographic and theoretical investigation of selected diorganotin systems. J Phy Chem A 102:2472–2482
Willem R, Verbruggen I, Gielen M, Biesemans M, Mahieu B, Basu Baul TS, Tiekink ERT (1998) Correlating Mossbauer and solution- and solid-state 117Sn NMR data with X-ray diffraction structural data of triorganotin 2-[(E)-2-(2-Hydroxy-5-methylphenyl)-1-diazenyl]benzoates. Organometallics 17:5758–5766
Dakternieks D, Duthie A, Smyth DR, Stapleton CPD, Tiekink ERT (2003) Steric control over molecular structure and supramolecular association exerted by tin- and ligand-bound groups in diorganotin carboxylates. Organometallics 22:4599–4603
Prabusankar G, Murugavel R (2004) Hexameric organotincarboxylates with cyclic and drum structures. Organometallics 23:5644–5647
Gielen M, Tiekink ERT (eds) (2005) Metallotherapeutic drug and metal-based diagnostic agents: 50Sn Tin compounds and their therapeutic potential. Wiley, Chichester, pp 421–439 (and references therein)
Basu Baul TS, Basu S, de Vos D, Linden A (2009) Amino acetate functionalized Schiff base organotin(IV) complexes as anticancer drugs: synthesis, structural characterization, and in vitro cytotoxicity studies. Invest New Drugs 27:419–431
Basu Baul TS, Masharing C, Ruisi G, Jirásko R, Holčapek M, de Vos D, Wolstenholme D, Linden A (2007) Self-assembly of extended Schiff base amino acetate skeletons, 2-{[(2Z)-(3-hydroxy-1-methyl-2-butenylidene)]amino}phenylpropionate and 2-{[(E)-1-(2-hydroxyaryl)alkylidene]amino}phenylpropionate skeletons incorporating organotin(IV) moieties: synthesis, spectroscopic characterization, crystal structures, and in vitro cytotoxic activity. J Organomet Chem 692:4849–4862
Basu Baul TS, Paul A, Pellerito L, Scopelliti M, Singh P, Verma P, de Vos D (2009) Triphenyltin(IV) 2-[(E)-2-(aryl)-1-diazenyl]benzoates as anticancer drugs: synthesis, structural characterization, in vitro cytotoxicity and study of its influence towards the mechanistic role of some key enzymes. Invest New Drugs. doi:10.1007-s10637-009-9293-x
Corral E, Hotze ACG, den Dulk H, Leczkowska A, Rodger A, Hannon MJ, Reedijk J (2009) Ruthenium polypyridyl complexes and their modes of interaction with DNA: is there a correlation between these interactions and the antitumor activity of the compounds? J Biol Inorg Chem 14:439–448
Basu Baul TS, Rynjah W, Willem R, Biesemans M, Verbruggen I, Holčapek M, de Vos D, Linden A (2004) Dibutyltin(IV) complexes of the 5-[(E)-2-(Aryl)-1-diazenyl]-2-hydroxybenzoic acid ligand: an investigation of structures by X-ray diffraction, solution and solid state tin NMR, electrospray ionisation MS and assessment of in vitro cytotoxicity. J Organomet Chem 689:4691–4701
Basu Baul TS, Rynjah W, Rivarola E, Lyčka A, Holčapek M, Jirásko R, de Vos D, Butcher RJ, Linden A (2006) Synthesis and characterization of bis[dicarboxylatotetraorganodistannoxane] units involving 5-[(E)-2-(aryl)-1-diazenyl]-2-hydroxybenzoic acids: an investigation of structures by X-ray diffraction, NMR, electrospray ionization MS and assessment of in vitro cytotoxicity. J Organomet Chem 691:4850–4862
Basu Baul TS, Paul A, Arman HD, Tiekink ERT (2008) 2-[(E)-(5-tert-Butyl-2-hydroxyphenyl)diazenyl]benzoic acid. Acta Crystallogr Sect. E 64:o2125
Boyd MR (1989) Status of the NCI preclinical antitumor drug discovery screen. Principles and practice of oncology, vol 3. Lippincott, Philadelphia, pp 1–12
Keepers YP, Pizao PR, Peters GJ, Ark-Otte JV, Winograd B, Pinedo HM (1991) Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur J Cancer 27:897–900
Higashi T (1995) ABSCOR. Rigaku Corporation, Tokyo, Japan
Beurskens PT, Admiraal G, Beurskens G, Bosman WP, García-Granda S, Smits JMM, Smykalla C (1992) The DIRDIF program system, technical report of the Crystallography Laboratory. University of Nijmegen, The Netherlands
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A A64:112–122
Johnson CK (1976) ORTEP II, Report ORNL-5136. Oak Ridge National Laboratory, Oak Ridge, TN
Brandenburg K (2006) DIAMOND. Crystal impact GbR, Bonn, Germany
Stewart JJP (1989) Optimization of parameters for semiempirical methods: I. Method. J Comput Chem 10:209–220
Stewart JJP (1989) Optimization of parameters for semiempirical methods: II. Applications. J Comput Chem 10:221–264
Stewart JJP (1991) Optimization of parameters for semiempirical methods: III. Extension of PM3 to Be, Mg, Zn, Ga, Ge, As, Se, Cd, In, Sn, Sb, Te, Hg, Tl, Pb, and Bi. J Comput Chem 12:320–341
Stewart JJP (2004) Optimization of parameters for semiempirical methods IV. Extension of MNDO, AM1, and PM3 to more main group elements. J Mol Model 10:155–164
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213
VandeVondele J, Krack M, Mohamed F, Parrinello M, Chassaing T, Hutter J (2005) Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comp Phys Comm 167:103–128
ArgusLab 4.0.1: Thompson MA, Planaria Software LLC, Seattle, WA, http://www.argusLab.com
Protein Data Bank, <http://www.rcsb.org/pdb/>
Wang R, Lai L, Wang S (2002) Further development and validation of empirical scoring functions for structure-based binding affinity prediction. J Comput Aided Mol Des 16:11–26
Basu Baul TS, Pyke SM, Sarma KK, Tiekink ERT (1996) Crystal and molecular structure of aquatriphenyltin 2-(3-formyl-4-hydroxyphenylazo)benzoate. Main Group met. Chem. 19:807–814
Basu Baul TS, Dhar S, Pyke SM, Tiekink ERT, Rivarola E, Butcher R, Smith FE (2001) Synthesis and characterization of triorganotin(IV) complexes of 5-[(E)-2-(aryl)-1-diazenyl]-2-hydroxybenzoic acids. Crystal and molecular structures of a series of triphenyltin 5-[(E)-2-(aryl)-1-diazenyl]-2-hydroxybenzoates (aryl = phenyl, 2-methylphenyl, 3-methylphenyl and 4-methoxyphenyl). J Organomet Chem 633:7–17
Basu Baul TS, Dhar S, Rivarola E, Smith FE, Butcher R, Song X, McCain M, Eng G (2003) Synthesis and characterization of some dibutylbis{5-[(E)-2-(aryl)-1-diazenyl]-2-hydroxybenzoato}tin(IV) compounds. Toxicity studies of di- and tri-organotin complexes on the second instar of Aedes aegypti mosquito larvae. Appl Organomet Chem 17:261–267
Basu Baul TS, Rynjah W, Rivarola E, Pettinary C, Linden A (2005) Synthesis and characterization of the first diorganotin(IV) complexes containing mixed arylazobenzoic acids and having skew trapezoidal bipyramidal geometry. J Organomet Chem 690:1413–1421
Otera J, Hinoishi T, Kawabe Y, Okawara R (1981) 119Sn, 13C, and 1H NMR studies on six-coordinate dimethyltin bis(chelate) compounds. Chem Lett 10:273–274
Lockhart TP, Manders WF (1986) Structure determination by NMR spectroscopy. Dependence of |2 J(119Sn, 1H)| on the Me-Sn-Me angle in methyltin(IV) compounds. Inorg Chem 25:892–895
Lockhart TP (1988) Solution and solid-state structures of di-n-butyltin 3-thiopropionate. X-ray crystal structure determination of the cyclic hexamer. Organometallics 7:1438–1443
Barbieri R, Huber F, Pellerito L, Ruisi G, Silvestri A (1998) In: Smith PJ (ed) Chemistry of tin: 119Sn Mössbauer studies on tin compounds. Blackie, London, pp 496–540
Greenwood NN, Gibb TC (1971) Mössbauer spectroscopy. Chapman & Hill, London
Donaldson JD, Grimes SM, Pellerito L, Girasolo MA, Smith PJ, Cambria A, Famà M (1987) Thermal behaviour, 119Sn Mössbauer and IR spectroscopic studies of some diorganotin(IV)carbohydrates. Polyhedron 6:383–386
Sham TK, Bancroft GM (1975) Tin-119 Mossbauer quadrupole splittings for distorted dimethyltin(IV) structures. Inorg Chem 14:2281–2283
Bancroft GM, Kumar Das VG, Sham TK, Clark MG (1976) Additive model for 119Sn Mössbauer quadrupole splitting in five-co-ordinate organotin(IV) compounds. J Chem Soc, Dalton Trans 643–654
Szorcsik A, Nagy L, Sletten J, Szalontai G, Kamu E, Fiore T, Pellerito L, Kalman E (2004) Preparation and structural studies on dibutyltin(IV) complexes with pyridine mono- and dicarboxylic acids. J Organomet Chem 689:1145–1154
Szorcsik A, Nagy L, Deak A, Scopelliti M, Fekete ZA, Csaszar A, Pellerito C, Pellerito L (2004) Preparation and structural studies on the tBu2Sn(IV) complexes with aromatic mono- and dicarboxylic acids containing hetero {N} donor atom. J Organomet Chem 689:2762–2769
Pelizzi C, Pelizzi G, Tarasconi P (1983) Synthesis and characterization by IR spectroscopy and x-ray diffraction of a quinazoline-complex of dibutyldichlorotin(IV). Polyhedron 2:145–147
Di Nicola C, Galindo A, Hanna JV, Marchetti F, Pettinari C, Pettinari R, Rivarola E, Skelton BW, White AH (2005) Synthesis and spectroscopic and X-ray structural characterization of R2SnIV-oxydiacetate and -iminodiacetate complexes. Inorg Chem 44:3094–3102
Xueqing S, Zhiqiang Y, Qinglan X, Jinshan L (1998) Synthesis, structures and in vitro antitumor activity of some germanium-substituted di-n-butyltin dipropionates. J Organomet Chem 566:103–110
Ng SW, Chen W, Zainudin A, Kumar Das VG, Yip W-H, Wang R-J, Mak TCW (1991) Crystal structure of trans-C2SnO5 pentagonal bipyramidal dibutylbis(phenylacetato)tin(IV) hydrate. J Crystallogr Spectros Res 21:39–43
Dakternieks D, Kuan FS, Tiekink ERT (2001) X-ray structure of di(Acetato)-aqua-di(cyclohexyl)tin(IV). Main Group Met Chem 24:291–292
Mahon MF, Molloy KC, Stanley JE, Rankin DWH, Robertson HE, Johnson BF (2005) Atmospheric pressure deposition of fluorine-doped SnO2 thin films from organotin fluorocarboxylate precursors. Appl Organomet Chem 19:658–671
Molloy KC (1989) In: Hartley FR (ed) Bioorganotin compounds in the chemistry of metal carbon bond, vol 5. Wiley, New York (Chapter 11)
Hook JM, Linahan BM, Taylor RL, Tiekink ERT, Von Gorkom N, Webster LK (1994) Phenyltin diethyldithiocarbamates: solid state and solution structures and in vitro anti-tumour activity. Main Group Met Chem 17:293–311
Willem R, Dalil H, Biesemans M, Martins JC, Gielen M (1999) The reaction of di-n-butyltin oxide with hexafluoro-2, 2-bis(4-carboxyphenyl)propane. Appl Organomet Chem 13:605–608
Danish M, Alt Helmut G, Badshah A, Ali S, Mazhar M, Nazar-ul-Islam (1995) Organotin esters of 3-(2-furanyl)-2-propenoic acid: their characterization and biological activity. J Organomet Chem 486:51–56
Acknowledgements
The financial support of the Department of Science & Technology, New Delhi, India (Grant No.SR/S1/IC-03/2005,TSBB and SR/S1/OC-11A/2006, PS), of the Università degli Studi di Palermo, Italy (Grants ORPA079E5M and ORPA0737W2) and the University Grants Commission, New Delhi, India through SAP-DSA, Phase-III, are gratefully acknowledged. The in vitro cytotoxicity experiments were carried out by Ms. P. F. van Cuijk in the Laboratory of Translational Pharmacology, Department of Medical Oncology, Erasmus Medical Center, Rotterdam, The Netherlands, under the supervision of Dr. E. A. C. Wiemer and Prof. Dr. G. Stoter.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Unit cell contents viewed in projection down the c-axis for 1 (GIF 138 kb)
Rights and permissions
About this article
Cite this article
Basu Baul, T.S., Paul, A., Pellerito, L. et al. Dibutyltin(IV) complexes containing arylazobenzoate ligands: chemistry, in vitro cytotoxic effects on human tumor cell lines and mode of interaction with some enzymes. Invest New Drugs 29, 285–299 (2011). https://doi.org/10.1007/s10637-009-9360-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9360-3