Abstract
The CO oxidation reactivity of negatively and positively charged isolated cuboctahedron (c-Oh) Au13 and Au12Ag nanoparticles is investigated using density functional theory calculations. Charging the nanoparticles modifies the structural stability of the Au13 and Au12Ag nanoparticles as well as the electron distribution in the core and shell atoms. An Ag-doping in gold (Au) clusters improves CO or O2 adsorption on Au12Ag cluster. For Au13 cluster, CO preadsorption increases the capacity of CO and O2 coadsorption, but the result is opposite for Au12Ag cluster. The neutral Au13 and Au12Ag clusters exhibit relatively poor reactivity for CO oxidation, while the reactivity is enhanced significantly by excess electrons. In comparisons of the results of CO oxidation on Ag- and un-doped Au nanoparticles, we discover Ag-doping in Au cluster surely decreases first energy barrier (Ea), and increases slightly second energy barrier (Eb). This work provides a fundamental insight into how the excess charges affect the adsorption activity and how the Ag-doping in Au clusters adjusts the catalytic activity for Ag- or un-doped c-Oh Au clusters.
Graphical Abstract
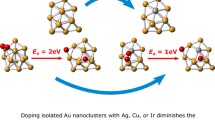
Reaction pathways for CO + O2 → CO2 + O associated with Au13 and Au12Ag clusters. Here, * denotes the adsorbed species on an Au13 or Au12Ag cluster. The reactivity of CO oxidation on Au nanoparticles is enhanced significantly by excess electrons. An Ag-doping in Au cluster improves CO or O2 adsorption on Au12Ag cluster. Ag-doping in Au clusters decreases first energy barrier (Ea), and increases slightly second energy barrier (Eb). Ag-doping in Au nanoparticles weakens C–Au bond at CO + O2 coadsorption state, and strengths CO–O bonds at transition states and intermediate state.








Similar content being viewed by others
References
Arenz M, Mayrhofer KJJ, Stamenkovic V, Blizanac BB, Tomoyuki T, Ross PN, Markovic NM (2005) J Am Chem Soc 127:6819–6829
Jiang T, Mowbray DJ, Dobrin S, Falsig H, Hvolbæk B, Bligaard T, Nørskov JK (2009) J Chem Phys C 113:10548–10553
Janssens TVW, Carlsson A, Puig-Molina A, Clausen BSJ (2006) J Catal 240:108–113
Wang Y, Gong XG (2006) J Chem Phys 125:124703
Gao Y, Shao N, Bulusu S, Zeng XC (2008) J Phys Chem C 112:8234–8238
Gao Y, Shao N, Pei Y, Chen ZF, Zeng XC (2011) ACS Nano 5:7818–7829
Yoon B, Koskinen P, Huber B, Kostko O, von Issendorff B, Häkkinen H, Moseler M, Landman U (2007) ChemPhysChem 8:157
Yoon B, Häkkinen H, Landman U (2003) J Phys Chem A 107:4066
Wu X, Senapati L, Nayak SK, Selloni A, Hajaligol M (2002) J Chem Phys 117:4010
Xie YP, Gong XG (2010) J Chem Phys 132:244302
Socaciu LD, Hagen J, Bernhardt TM, Wöste L, Heiz U, Häkkinen H, Landman U (2003) J Am Chem Soc 125:10437–10445
Yuan DW, Zeng Z (2004) J Chem Phys 120:6574
Johnson GE, Reilly NM, Tyo EC, Castleman AW (2008) J Phys Chem C 112:9730
Yoon B, Häkkinen H, Landman U, Wörz AS, Antonietti J, Abbet S, Judai K, Heiz U (2005) Science 307:403
Hagen J, Socaciu LD, Elijazyfer M, Heiz U, Bernhardt TM, Woste L (2002) Phys Chem Chem Phys 4:1707
Wallace WT, Whetten RL (2002) J Am Chem Soc 124:7499
Tang DY, Hu CW (2011) J Phys Chem Lett 2:2972–2977
Molayem M, Grigoryan VG, Springborg M (2011) J Phys Chem C 115:22148–22162
Jirkovský JS, Panas I, Ahlberg E, Halasa M, Romani S, Schiffrin DJ (2011) J Am Chem Soc 133:19432–19441
Molina LM, Hammer B (2005) J Catal 233:399
Price SWT, Speed JD, Kannan P, Russell AE (2011) J Am Chem Soc 133:19448–19458
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Delley B (2002) Phys Rev B 66:155125
Delley B (1990) J Chem Phys 92:508–517
Delley B (2000) J Chem Phys 113:7756–7764
Henkelman G, Uberuaga BP, Jonsson HA (2000) J Chem Phys 113:9901–9904
Henkelman G, Jonsson H (2000) J Chem Phys 113:9978–9985
Chen FY, Johnston RL (2008) Acta Mater 56:2374
Santos E, Schmickler W (2006) ChemPhysChem 7:2282–2285
Stamenkovic VR, Mun BS, Arenz M, Mayrhofer KJJ, Lucas CA, Wang G, Ross PN, Markovic NM (2007) Nat Mater 6:241–247
Häkkinen H, Moseler M, Landman U (2002) Phys Rev Lett 89:033401
Fielicke A, Gruene P, Meijer G, Rayner DM (2009) Surf Sci 603:1427–1433
Hammer B, Nørskov JK (2000) Adv Catal 45:71–129
Kim DH, Shin K, Lee HM (2011) J Phys Chem C 115:24771–24777
Falsig H, Hvolbæk B, Kristensen IS, Jiang T, Bligaard T, Christensen CH, Nørskov J (2008) Angew Chem Int Ed 47:4835–4839
Wang CM, Fan KN, Liu ZP (2007) J Am Chem Soc 129:2642
Molina LM, Hammer B (2003) Phys Rev Lett 90:206102
Molina LM, Rasmussen MD, Hammer BJ (2004) Chem Phys 120:7673
An W, Pei Y, Zeng XC (2008) Nano Lett 8:195–202
Gao Y, Shao N, Pei Y, Zeng XC (2010) Nano Lett 10:1055–1062
Zhang C, Michaelides A, King DA, Jenkins SJ (2008) J Chem Phys 129:197408
Acknowledgments
The authors are grateful for the support of this research by the National Natural Science Foundation of People’s Republic of China (No. 50971100/E010503 and No. 51271148) and the Natural Science Foundation of Shaanxi Province in People’s Republic of China (No. 2010JK917 and No. 2012SXTS05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, W., Chen, F. CO Oxidation on the Ag-Doped Au Nanoparticles. Catal Lett 143, 84–92 (2013). https://doi.org/10.1007/s10562-012-0922-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-012-0922-1