Abstract
Objective
To determine: (1) the correlation of prostate cancer incidence and mortality with groundwater boron and selenium concentrations; and (2) the impact of boron on prostate cancer cell proliferation during co-treatment with alternative chemo-preventative agents, along with boron pre-treatment effects on cell sensitivity to ionizing radiation.
Methods
For regression analysis, data on prostate cancer incidence and mortality were obtained from the Texas Cancer Registry, while groundwater boron and selenium concentrations were derived from the Texas Water Development Board. Cultured DU-145 prostate cancer cells were used to assess the impact of boric acid on cell proliferation when applied in combination with selenomethionine and genistein, or preceding radiation exposure.
Results
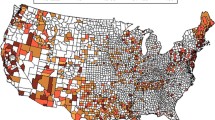
Groundwater boron levels correlated with a decrease in prostate cancer incidence (R = 0.6) and mortality (R = 0.6) in state planning regions, whereas selenium did not (R = 0.1; R = 0.2). Growth inhibition was greater during combined treatments of boric acid and selenomethionine, or boric acid and genistein, versus singular treatments. 8-day boric acid pre-exposure enhanced the toxicity of ionizing radiation treatment, while dose-dependently decreasing the expression of anti-apoptotic protein Bcl-2.
Conclusions
Increased groundwater boron concentrations, across the state of Texas, correlate with reduced risk of prostate cancer incidence and mortality. Also, boric acid improves the anti-proliferative effectiveness of chemo-preventative agents, selenomethionine and genistein, while enhancing ionizing radiation cell kill.




Similar content being viewed by others
References
Jemal A, Murray T, Ward E, et al (2005). Cancer statistics. CA Cancer J Clin 55(1):10–30
Willis MS, Wians FH (2004) The role of nutrition in preventing prostate cancer: a review of the proposed mechanism of action of various dietary substances. Clinica Chimica Acta 330: 57–83
Djavan B, Zlotta A, Schulman C, et al (2004) Chemotherapeutic prevention studies of prostate cancer. J Urol 171(2 Pt 2): S10–S13
Klein EA, Thompson IM, Lippman SM, et al (2001) SELECT: the next prostate cancer prevention trial. Selenium and Vitamin E Cancer Prevention Trial. J Urol 166(4): 1311–1315
Cui Y, Winton MI, Zhang ZF, et al (2004) Dietary boron intake and prostate cancer risk. Oncol Rep 11(4): 887–892
Gallardo-Williams MT, Chapin RE, King PE, et al (2004) Boron supplementation inhibits the growth and local expression of IGF-1 in human prostate adenocarcinoma (LNCaP) tumors in nude mice. Toxicol Pathol 32: 73–78
Gallardo-Williams MT, Maronpot RR, Wine RN, Brunssen SH, Chapin RE (2003) Inhibition of the enzymatic activity of prostate specific antigen by BA and 3-nitrophenyl boronic acid. Prostate 54: 44–49
Barranco WT, Eckhert CD (2004) BA inhibits human prostate cancer cell proliferation. Cancer Lett 216(1): 21–9
Kobayashi M, Matoh T, Azuma J (1996) Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol 110(3): 1017–1020
O’Neill MA, Warrenfeltz D, Kates K, et al (1997) Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. In vitro conditions for the formation and hydrolysis of the dimer. J Biol Chem 271(37): 22923–22930
Takano J, Noguchi K, Yasumori M, et al (2002) Arabidopsis boron transporter for xylem loading. Nature 420(6913): 337–340
Anderson DL, Cunningham WC, Lindstrom TR (1994) Concentrations and intakes of H, B, S, K Na, Cl, and NaCl in foods. J Food Comp Anal 7: 59–82
Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. A report of the panel on micronutrients, subcommittees on upper reference levels of nutrients and of interpretation and use of dietary reference intakes, and the standing committee on the scientific Evaluation of dietary reference Intakes. (2001) Food and Nutrition Board, Institute of Medicine, National Academy Press, Washington, D.C., p C-13
Hudak PF (2004) Boron and selenium contamination in south Texas groundwater. J Environ Sci Health A Tox Hazard Subst Environ Eng 39(11–12):2827–2834
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. U.S. Geological Survey Water Supply Paper 2254
http://www.tcc.state.tx.us/tcplan/goal2/goal2_obje_frames.html
http://www.dshs.state.tx.us/tcr, accessed May 31, 2005
http://www.twdb.state.tx.us, accessed May 31, 2005
Panel on Dietary Reference Intakes for Electrolytes and Water (2004) Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. National Academies Press, Washington, D.C., p 494.
Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids (2000) Selenium. National Academies Press, Washington, D.C., p. 284.
Clark LC, Dalkin B, Krongrad A, et al (1998) Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol 81: 730–734
Eckhert CD, Lockwood MK, Shen B (1993) Influence of selenium on the microvascular circulation of the retina. Microvas Res 45: 74–82
Thornber JM, Eckhert CD (1984) Protection against sucrose induced retinal capillary damage in the Wistar rat. J Nutr 114:1070−1075
Bischof M, Abdollahi A, Gong P, et al (2004) Triple combination of irradiation, chemotherapy (pemetrexed), and VEGFR inhibition (SU5416) in human endothelial and tumor cells. Int J Radiat Oncol Biol Phys 60(4):1220–1232
Yan SX, Ejima Y, Sasaki R, et al (2004) Combination of genistein with ionizing radiation on androgen-independent prostate cancer cells. Asian J Androl 6(4): 285–290
Husbeck B, Peehl DM, Knox SJ (2005) Redox modulation of human prostate carcinoma cells by selenite increases radiation-induced cell killing. Free Radic Biol Med 38(1):50–57
Bendel P (2005) Biomedical applications of 10B and 11B NMR. NMR Biomed 18(2): 74–82
Rudner J, Jendrossek V, Belka C (2002) New insights in the role of Bcl-2 Bcl-2 and the endoplasmic reticulum. Apoptosis 7(5): 441–447
Rosser CJ, Reyes AO, Vakar-Lopez F et al (2003) Bcl-2 is significantly overexpressed in localized radio-recurrent prostate carcinoma, compared with localized radio-naive prostate carcinoma. Int J Radiat Oncol Biol Phys 56(1): 1–6
Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM (2004) Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene 23(8): 1599–1607
Campbell MJ, Dawson M, Koeffler HP (1998) Growth inhibition of DU-145 prostate cancer cells by a Bcl-2 antisense oligonucleotide is enhanced by N-(2-hydroxyphenyl) all-trans retinamide. Br J Cancer 77(5): 739–744
Raffo A, Lai JC, Stein CA, et al (2004) Antisense RNA down-regulation of bcl-2 expression in DU145 prostate cancer cells does not diminish the cytostatic effects of G3139 (Oblimersen). Clin Cancer Res May 10(9): 3195–3206
Acknowledgments
We thank Dr. Allan Pantuck and Randy Kallilew for their expertise concerning the culturing of prostate cancer cells, along with Kurt Hafer and Cecelia Chan for aiding in the administration of ionizing radiation. Funding for this research was provided by: DOD prostate idea grant DAMD17–03-1-0067 (CD Eckhert) and UC TRS&TP (WT Barranco). In the spirit of full disclosure, we declare that since 1997 the National Institutes of Health has without exception declined funding of all grant applications submitted by CE on the role of boron in biology or cancer with recent applications triaged prior to full review.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10552-007-9023-7.
Rights and permissions
About this article
Cite this article
Barranco, W.T., Hudak, P.F. & Eckhert, C.D. Evaluation of ecological and in vitro effects of boron on prostate cancer risk (United States). Cancer Causes Control 18, 71–77 (2007). https://doi.org/10.1007/s10552-006-0077-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10552-006-0077-8