Abstract
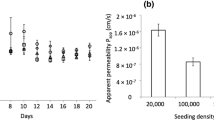
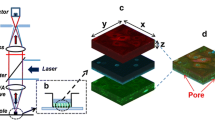
We describe a compartmentalized microdevice specifically designed to perform permeability studies across a model of lung barrier. Epithelial cell barriers were reproduced by culturing Calu-3 cells at the air-liquid interface (AIC) in 1 mm2 microwells made from a perforated glass slide with an embedded porous membrane. We created a single basolateral reservoir for all microwells which eliminated the need to renew the growth medium during the culture growth phase. To perform drug permeability studies on confluent cell layers, the cell culture slide was aligned and joined to a collection platform consisting in 35 μL collection reservoirs connected at the top and bottom with microchannels. The integrity and functionality of the cell barriers were demonstrated by measurement of trans-epithelial electrical resistance (TEER), confocal imaging and permeability assays of 14C-sucrose. Micro-cell barriers were able to form confluent layers in 1 week, demonstrating a similar bioelectrical evolution as the Transwell systems used as controls. Tight junctions were observed throughout the cell-cell interfaces, and the low permeability coefficients of 14C-sucrose confirmed their functional presence, creating a primary barrier to the diffusion of solutes. This microdevice could facilitate the monitoring of biomolecule transport and the screening of formulations promoting their passage across the pulmonary barrier, in order to select candidates for pulmonary administration to patients.




Similar content being viewed by others
References
R.U. Agu, M.I. Ugwoke, M. Armand et al., The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir. Res. 2, 198–209 (2001)
L. Baginski, F. Tewes, S.T. Buckley et al., Investigations into the fate of inhaled salmon calcitonin at the respiratory epithelial barrier. Pharm. Res. 29, 332–341 (2012)
R. Baudoin, L. Griscom, M. Monge et al., Development of a renal microchip for in vitro distal tubule models. Biotechnol. Prog. 23, 1245–1253 (2007)
R. Booth, H. Kim, Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 12, 1784–1792 (2012)
E.M.M. Del Valle, M.A. Galan, R.G. Carbonell, Drug delivery technologies: the way forward in the new decade. Ind. Eng. Chem. Res. 48, 2475–2486 (2009)
N.J. Douville, P. Zamankhan, Y.-C. Tung et al., Combination of fluid and solid mechanical stresses contribute to cell death and detachment in a microfluidic alveolar model. Lab Chip 11, 609–619 (2011)
C. Ehrhardt, J. Fiegel, S. Fuchs et al., Drug absorption by the respiratory mucosa: cell culture models and particulate drug carriers. J. Aerosol Med. 15, 131–139 (2002)
M. Felder, P. Sallin, L. Barbe et al., Microfluidic wound-healing assay to assess the regenerative effect of HGF on wounded alveolar epithelium. Lab Chip 12, 640–646 (2012)
J. Fiegel, C. Ehrhardt, U.F. Schaefer et al., Large porous particle impingement on lung epithelial cell monolayers–toward improved particle characterization in the lung. Pharm. Res. 20, 788–796 (2003)
B.I. Florea, M.L. Cassara, H.E. Junginger et al., Drug transport and metabolism characteristics of the human airway epithelial cell line Calu-3. J. Control. Release 87, 131–138 (2003)
B.I. Florea, M. Thanou, H.E. Junginger et al., Enhancement of bronchial octreotide absorption by chitosan and N-trimethyl chitosan shows linear in vitro/in vivo correlation. J. Control. Release 110, 353–361 (2006)
B. Forbes, Human airway epithelial cell lines for in vitro drug transport and metabolism studies. Pharm. Sci. Technol. Today 3, 18–27 (2000)
K.A. Foster, M.L. Avery, M. Yazdanian et al., Characterization of the Calu-3 cell line as a tool to screen pulmonary drug delivery. Int. J. Pharm. 208, 1–11 (2000)
D. Gao, H. Liu, J.M. Lin et al., Characterization of drug permeability in Caco-2 monolayers by mass spectrometry on a membrane-based microfluidic device. Lab Chip 13, 978–985 (2013)
R.W. Godfrey, Human airway epithelial tight junctions. Microsc. Res. Tech. 38, 488–499 (1997)
C.I. Grainger, L.L. Greenwell, D.J. Lockley et al., Culture of calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm. Res. 23, 1482–1490 (2006)
M. Haghi, P.M. Young, D. Traini et al., Time- and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev. Ind. Pharm. 36, 1207–1214 (2010)
D. Huh, H. Fujioka, Y.C. Tung et al., Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc. Natl. Acad. Sci. 104, 18886–18891 (2007)
D. Huh, B.D. Matthews, A. Mammoto et al., Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010)
Y. Imura, Y. Asano, K. Sato et al., A microfluidic system to evaluate intestinal absorption. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 25, 1403–1407 (2009)
H. Kimura, T. Yamamoto, H. Sakai et al., An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab Chip 8, 741 (2008)
A. Lewis, F. Jordan, L. Illum, CriticalSorb™: enabling systemic delivery of macromolecules via the nasal route. Drug Deliv. Transl. Res. 3, 26–32 (2013)
N.R. Mathias, J. Timoszyk, P.I. Stetsko et al., Permeability characteristics of calu-3 human bronchial epithelial cells: in vitro-in vivo correlation to predict lung absorption in rats. J. Drug Target. 10, 31–40 (2002)
L. Müller, M. Riediker, P. Wick et al., Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J. R. Soc. Interface. 7, 27–41 (2010)
S. Mura, H. Hillaireau, J. Nicolas et al., Biodegradable nanoparticles meet the bronchial airway barrier: how surface properties affect their interaction with mucus and epithelial cells. Biomacromolecules 12, 4136–4143 (2011a)
S. Mura, H. Hillaireau, J. Nicolas et al., Influence of surface charge on the potential toxicity of PLGA nanoparticules towards Calu-3 cells. Int. J. Nanomedicine 6, 2591–2605 (2011b)
D.D. Nalayanda, Q. Wang, W.B. Fulton et al., Engineering an artificial alveolar-capillary membrane: a novel continuously perfused model within microchannels. J. Pediatr. Surg. 45, 45–51 (2010)
J.S. Patton, Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 19, 3–36 (1996)
J.S. Patton, C.S. Fishburn, J.G. Weers, The lungs as a portal of entry for systemic drug delivery. Proc. Am. Thorac. Soc. 1, 338–344 (2004)
I. Pezron, R. Mitra, D. Pal et al., Insulin aggregation and asymmetric transport across human bronchial epithelial cell monolayers (Calu-3). J. Pharm. Sci. 91, 1135–1146 (2002)
B. Prabhakarpandian, M.-C. Shen, J.B. Nichols et al., SyM-BBB: a microfluidic blood brain barrier model. Lab Chip 13, 1093–1101 (2012)
B.M. Rothen-Rutishauser, S.G. Kiama, P. Gehr, A three-dimensional cellular model of the human respiratory tract to study the interaction with particles. Am. J. Respir. Cell Mol. Biol. 32, 281–289 (2005)
J. Shao, L. Wu, J. Wu et al., A microfluidic chip for permeability assays of endothelial monolayer. Biomed. Microdevices 12, 81–88 (2010)
A. Stentebjerg-Andersen, I.V. Notlevsen, B. Brodin et al., Calu-3 cells grown under AIC and LCC conditions: implications for dipeptide uptake and transepithelial transport of substances. Eur. J. Pharm. Biopharm. 78, 19–26 (2011)
H. Tavana, P. Zamankhan, P.J. Christensen et al., Epithelium damage and protection during reopening of occluded airways in a physiologic microfluidic pulmonary airway model. Biomed. Microdevices 13, 731–742 (2011)
D. Teijeiro-Osorio, C. Remuñán-López, M.J. Alonso, Chitosan/cyclodextrin nanoparticles can efficiently transfect the airway epithelium in vitro. Eur. J. Pharm. Biopharm. 71, 257–263 (2009)
R. Tréhin, U. Krauss, A.G. Beck-Sickinger et al., Cellular uptake but Low permeation of human calcitonin-derived cell penetrating peptides and tat(47–57) through well-differentiated epithelial models. Pharm. Res. 21, 1248–1256 (2004)
D. Vllasaliu, R. Fowler, M. Garnett et al., Barrier characteristics of epithelial cultures modelling the airway and intestinal mucosa: a comparison. Biochem. Biophys. Res. Commun. 415, 579–585 (2011)
D. Vllasaliu, C. Alexander, M. Garnett et al., Fc-mediated transport of nanoparticles across airway epithelial cell layers. J. Control. Release 158, 479–486 (2012)
S. Wang, M.S.S. Chow, Z. Zuo, An approach for rapid development of nasal delivery of analgesics—Identification of relevant features, in vitro screening and in vivo verification. Int. J. Pharm. 420, 43–50 (2011)
M.-H. Wu, S.B. Huang, G.-B. Lee, Microfluidic cell culture systems for drug research. Lab Chip 10, 939–956 (2010)
L.Y. Yeo, H.C. Chang, P.P.Y. Chan et al., Microfluidic devices for bioapplications. Small 7, 12–48 (2011)
J. Yeon, D. Na, K. Choi et al., Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed. Microdevices 14, 1–8 (2012)
E.W.K. Young, M.W.L. Watson, S. Srigunapalan, Technique for real-time measurements of endothelial permeability in a microfluidic membrane chip using laser-induced fluorescence detection. Anal. Chem. 82, 808–816 (2010)
Acknowledgments
The authors would like to thank Simona Mura (Institut Galien Paris Sud) for her kind help and expertise in Calu-3 cells culture, and Hélène Chacun (Institut Galien Paris Sud) for her technical assistance for radioactivity experiments. We are grateful to Gillian Barratt (Institut Galien Paris Sud) for proof-reading the English of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bol, L., Galas, JC., Hillaireau, H. et al. A microdevice for parallelized pulmonary permeability studies. Biomed Microdevices 16, 277–285 (2014). https://doi.org/10.1007/s10544-013-9831-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-013-9831-3