Abstract
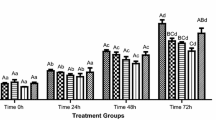
In a natural environment, seminal plasma provides spermatozoa with protection against reactive oxygen species. Storing semen in cooling conditions requires diluting it with various buffer solutions. Therefore, the protective role of seminal plasma is not sufficient enough. Semen obtained from five male specimens was diluted with the Kobayashi buffer solution at a 1:9 ratio. To determine the influence of antioxidants on semen storage, a buffer solution was used, as before, with the addition of 1 % albumin, 1 mM vitamin C, 1.5 mg ml−1 vitamin E, 5 mM sodium citrate, 5 mM glutathione and 5 mM cysteine. After the preparation of such tests, the parameters of spermatozoa motility were measured every 3–5 days, using the CASA system (Image House CRISMAS Company Ltd.). Among all used antioxidants, the best effects were observed after the addition of glutathione to semen. After 17 days of storage, the percentage of motile spermatozoa in the samples preserved with glutathione addition was 57 %, while without antioxidant addition, it was 44 %. Furthermore, the addition of cysteine and albumin also resulted in the lengthening of the life span of perch sperm cells. The presence of the remaining antioxidants (vitamins C and E, and sodium citrate) did not have any positive influence on spermatozoa viability, and in these samples, no motile spermatozoa were observed after 12 days of storage. Our data show that dilution of perch sperm with buffered solution might be a promising method for short-term storage.
Similar content being viewed by others
References
Bucak MN, Atessahin A, Varisli O, Yuce A, Tekin N, Akcay A (2007) The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen: microscopic and oxidative stress parameters after the freeze-thawing process. Theriogenology 67:1060–1067. doi:10.1016/j.theriogenology.2006.12.004
Ciereszko A, Dabrowski K (1995) Sperm quality and ascorbic acid concentration in rainbow trout semen are affected by dietary vitamin C: an across-season study. Biol Reprod 52:982–988
Glogowski J, Cejko BI, Kowalski R (2008) Krótkookresowe przechowywanie nasienia ryb—z tlenem czy bez? [Short-term milt storage—with or without oxygen?] In: Zakęś Z, Wolnicki J, Demska-Zakęś K, Kamiński R, Ulikowski D (eds) Biotechnologia w akwakulturze. Wyd IRŚ Olsztyn: 181–185 (in Polish)
Holt WV (2000) Fundamental aspects of sperm cryobiology: the importance of species and individual differences. Theriogenology 53(1):47–58. doi:10.1016/S0093-691X(99)00239-3
Kobayashi T, Fushiki S, Ueno K (2004) Improvement of sperm motility of sex-reversed male rainbow trout, Oncorhynchus mykiss, by incubation in high-pH artificial seminal plasma. Environ Biol Fish 69:419–425
Kowalski RK, Cejko BI, Sarosiek B, Demianowicz W, Glogowski J (2009) Przechowywanie nasienia ryb łososiowatych—przegląd metod i ich praktyczne zastosowanie w wylęgarniach. [The storage of salmon fish milt—the methods review and practical application]. In: Rozród, podchów, profilaktyka ryb łososiowatych i innych gatunków. Wyd. IRŚ, Olsztyn, pp 105–117 (in Polish)
Kowalski RK, Hliwa P, Cejko BI, Król J, Dietrich GJ, Stabiński R, Ciereszko A (2010) Sztuczny rozród stynki (Osmerus eperlanus) z zastosowaniem nasienia przechowywanego w warunkach chłodniczych. [Artificial reproduction of smelt (Osmerus eperlanus) with using short-term storage milt]. In: Rozród, podchów, profilaktyka ryb rzadkich i chronionych oraz innych gatunków. Wyd. IRŚ, Olsztyn, pp 121–129 (in Polish)
Mansour N, McNiven MA, Richrdson GF (2006) The effect of dietary supplementation with blueberry α-tocopherol or astaxanthin on oxidative stability of Arctic char (Salvelinus alpinus) semen. Theriogenology 66:373–382. doi:10.1016/j.theriogenology.2005.12.002
Martinez-Paramo S, Martinez-Pastor F, Martinez-Gonzales G, Herraez MP, Cabrita E (2009) Antioxidant status in fresh and cryopreserved sperm from gilthead sea bream (Sparrus aurata). The 2nd international workshop on biology of fish gametes, 8–12 September, Valencia, Spain. doi:10.1530/REP-10-0037
Metwally MAA, Fouad IM (2009) Effects of L-ascorbic acid on sperm viability in male grass carp (Ctenopharyngodon idellus). Global Vet 3:132–136
Moore AA (1987) Short-term storage and cryopreservation of walleye semen. Prog Fish Cult 49:40–43
Sanocka D, Kurpisz M (2004) Reactive oxygen species and sperm cells. Reprod Biol Endocrin 2:1–7. doi:10.1186/1477-7827-2-12
Sarosiek B, Cejko BI, Glogowski J, Kucharczyk, Żarski D, Targońska K, Kowalski RK (2011) Cryopreservation of ide (Leuciscus idus) milt in the presence of selected antioxidants. Conference: Aquacultue Europe, Rhodes, pp 976–977
Satterlield JR, Flickinger SA (1995) Factor influencing storage potential of preserved walleye semen. Prog Fish Cult 57(3):175–181
Telea A, Grozea I, Banatean D, Korbuly B, Dumitrescu G (2008) Pikeperch milt preservation on short and medium periods. Bull UASVM Animal Sci Biotechnol 65(1–2):296–300
Thuwanut P, Chatdarong K, Techakumphu M, Axner E (2008) The effect of antioxidants on motility, viability, acrosome integrity and DNA integrity of frozen-thawed epididymal cat spermatozoa. Theriogenology 70:233–240. doi:10.1016/j.theriogenology.2008.04.005
Triwulanningsih E, Situmorang P, Sugiarti T, Sianturi RG, Kusumaningrum DA (2008) The effect of glutathione addition in sperm diluents on the quality of bovine chilled semen. Indonesian J Agric Sci 1(1):64–69
Acknowledgments
The presented study is supported by National Science Centre Grant N 311 515 640.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarosiek, B., Dryl, K., Kucharczyk, D. et al. Motility parameters of perch spermatozoa (Perca fluviatilis L.) during short-term storage with antioxidants addition. Aquacult Int 22, 159–165 (2014). https://doi.org/10.1007/s10499-013-9679-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9679-9