Abstract
Ultrasound approach has been used to assess morphology of the gonadal structure in the sex-reversed rainbow trout females (neomales) provided in the course of 11β-hydroxyandrostenedione treatment applied within gonadal differentiation period. Eighteen matured individuals (in range 38.0–48.6 cm of body length and 802–1644 g of body weight) were scanned using digital ultrasound apparatus (DP-6900 model) Mindray Ltd., during the spawning season. After screening, fishes were killed to validate the morphological configuration and position of the gonads in the body cavity. The favorable place for cross-sectional imaging of gonadal lobes was half of the distance between the pectoral and pelvic fins below lateral line of neomales. Most of the examined specimens (61 %) had properly shaped, paired testis. Moreover, presence of individuals with asymmetrical gonads (33 %) and one bisexual fish was confirmed. There were no differences between total volume of sampled semen and sperm motility, but statistically significantly different in sperm concentration within selected (with symmetrical and asymmetrical testis) groups of neomales. It was confirmed that ultrasonic imaging is an efficient and accurate method to determine the state of gonads of mature sex-reversed rainbow trout females during spawning season and offers the opportunity for non-invasive detection of any morphological anomalies in their gonads.
Similar content being viewed by others
Introduction
The production of an all-female population has an important economic benefit in salmonid commercial aquaculture (Donaldson 1996). Modern and efficient way to produce monosex female rainbow trout stocks is indirect method of feminization where gynogenesis and then masculinization of fishes with androgens are used (Feist et al. 1995). Masculinized females, called also neomales, have a male phenotype but retain the female genotype (XX), so all spermatozoa produced in their functional testes carry an X chromosome (Geffen and Evans 2000). Reproductive system of sex-reversed females due to hormonal therapy is often modified, including the structure of testicular lobes, as well as the presence or lack of spermatic ducts. Lack of spermatic ducts in sex-reversed females makes it necessary to kill the fish in order to collect semen during spawning season (Kuzminski and Dobosz 2010).
Ultrasonic imaging has proven to be useful as a non-invasive technique to determine the sex and maturity of many commercially valuable freshwater and marine fish species, for example, steelhead (anadromous rainbow trout—Oncorhynchus mykiss) (Evans et al. 2004), Atlantic salmon Salmo salar L. (Mattson 1991), cod Cadus morhua (Karlsen and Holm 1994), Atlantic halibut Hippoglossus hippoglossus L. (Shields et al. 1993), and stellate sturgeon Acipenser stellatus (Moghim et al. 2002). In addition to sex identification, ultrasound can also be used to distinguish immature from mature specimens based on the maximum diameter of the gonad (Blythe et al. 1994). Fish with large, well-developed gonads were readily identifiable as mature relative to immature specimens, which had small gonads. Even fish as small as the Pacific herring Clupea pallasi can be segregated by sex and maturational status with ultrasound (Bonar et al. 1989). Despite ultrasound use in identifying both sex and maturation in other fish species, the gonadal morphology of sex-reversed females of rainbow trout via ultrasound has yet to be reported in literature. Evaluation of gonadal structure is important for estimation of gamete quality before killing the neomales. Compared with normal males, the semen of sex-reversed females is characterized by a higher sperm concentration but a lower percentage of sperm motility which consequently can result in lower fertilization rates (Robles et al. 2003; Nynca et al. 2012).
In this study, ultrasound approach has been used to assess morphology of the gonadal structure in the sex-reversed rainbow trout females during spawning season. It has also been validated USG method to create/selected group of specimens with regular, properly formed testis that could be more effective during artificial spawning and consequently the production of an all-female population.
Materials and methods
The study material originated from Department of Salmonid Research in Rutki, Inland Fisheries Institute in Olsztyn, Poland. The matured sex-reversed females of rainbow trout (at age 3+) were obtained through gynogenesis and masculinization of fry in subsequent generations. Due to masculinization, the fish were treated with a dose of 20 ppm 11β-hydroxyandrostenedione (OHA) (Sigma-Aldrich Ltd.) delivered with the feed. Hormones were given to the fry from the start of exogenous feeding for 60 days (within gonadal differentiation period). The appropriate amount of hormone (diluted in 96 % ethanol) was added to the Bio-Optimal trout starter (BioMar, Denmark) using the spray method. The fish were fed ad libitum.
Eighteen individuals (in range 38.0–48.6 cm of body length and 802–1644 g of body weight) (Table 1) were scanned using digital ultrasound apparatus (DP-6900 model) Mindray Ltd., during the spawning season (March–May). Digital ultrasound scanner using a curvilinear probe of 3.5/5.0 and linear probe 7.5 MHz transducer frequency was employed in gonadal morphology of sex-reversed females of rainbow trout and to produce cross-sectional testes area images. Cross sections were made from a head of fish to the ventral fins below lateral line (Fig. 1).
The probe was capable of imaging up to 4 cm of tissue depth with a horizontal plane. The examination consisted of gently placing the ultrasound probe along each fish’s abdomen and then moving the probe along the abdominal surface until the gonads were located. Precise measurements of the testis were taken by use of an elliptical trackball function that is commonly found on ultrasound units. Once the gonads were located and a clear ultrasound image was obtained, pictures from each fish were recorded in a digital adaptor and stored for later viewing.
After screening, sex-reversed females were killed to validate the anatomical position of the gonads in the body cavity and credibility of USG readings. Gonads together with any spermatic ducts were isolated, macroscopic photographs with a NIKON CoolPix 4500 of the structure were performed, and then gonads were weighed (±0.1 g) in order to determine the gonadosomatic index (GSI). The GSI was calculated as the ratio of gonadal weight (Wg) to the total fish weight (Wt); GSI = Wg/Wt × 100 %.
The gonads were removed from decapitated fish and squeezed by hand to extract the milt. The milt was strained through a gauze to remove any testicular tissue. The sperm motility parameters were measured over a 15 s period, between 5 and 20 s post-activation time and analyzed using a Hobson Sperm Tracker (Hobson Vision Ltd, Baslow, UK) due to procedure described by Dietrich et al. (2005). Video recordings (two replicates per sample) were made using a microscope with a 10× negative phase objective and a Sony CCD black and white camera (SPT-M108CE). Sperm was activated at a dilution ratio of 1:500 with 1 mM CaCl2, 20 mM Tris, 30 mM glycine, 125 mM NaCl, pH 9.0 (Billard 1992) supplemented with 0.5 % bovine albumin. Sperm concentration was measured using NucleoCounter SP-100 (Chemometec, Denmark) as described by Nynca and Ciereszko (2009). Semen was diluted 100 times with a sperm immobilizing solution (100 mM NaCl, 40 mM KCl, 3 mM CaCl2, 1.5 mM, MgCl2, and 50 mM Tris, pH 8.5) and then 101 times with either Reagent S100 (total count) or the same immobilizing solution (non-viable count).
All data were expressed as mean ± SD. All analyses were performed at a significance level of 0.05. The percentage data were subjected to normalization by arcsine transformation. Data were analyzed using one-way ANOVA followed by Tukey’s test for post hoc comparison of means.
Results
The part of body close to the half of the distance between the pectoral fins and pelvic fins provided the most diagnostic images of sex-reversed females of rainbow trout testes (Fig. 1). However, other locations along the abdomen, for example, just posterior to the pelvic fins, also provided useful diagnostic images. In some circumstances, more than one area within the fish’s body cavity had to be examined before diagnostic images could be obtained. The imaging of visceral organs (e.g., liver, gas bladder) just posterior to the transverse septum served as landmarks for locating the gonads. Identification of visceral organs was an important first step in making gonad localization because these structures were present in every fish regardless of structure reproductive system. Each fish’s swim bladder consistently blocked ultrasound images near the posterior end of the gonad because acoustic waves reverberated off the swim bladder, thus distorting ultrasound imaging. Fortunately, gonad viewing was still possible because the majority of the gonad resides anterior to the swim bladder.
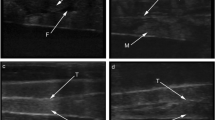
Most of the examined fishes (61 %) had properly shaped, paired testes—group S (Table 1; Fig. 2a). The remaining fish were recorded varied in terms of the structure of macroscopic gonadal lobes, as well as the presence or absence of spermatic ducts. Asymmetrical gonads (group A) were recorded in 6 specimens (33 % of fish) (Table 1). In this group of fish with atypical testis, lobes specimens were characterized by having a irregular gonad in size or divided by the number of fragments (Fig. 2b), single gonad (Fig. 2c). One bisexual fish with hermaphroditic gonads was found (Fig. 2d).
Values of GSI sex-reversed rainbow trout females in study groups (fish with symmetrical testis connected to spermatic ducts—group S, and fish with irregular shape of gonads—group A) were very similar, with means values above 3.0 % (Table 1). No differences between total volume of sampled milt were recorded. Generally sperm motility of neomales did not correlated with the macroscopic structure of their gonads, but sperm concentration was higher and statistically significantly different (p < 0.05) in group of sex-reversed females with asymmetrical gonads compare to other group of fishes (Table 1).
Discussion
Results indicated that ultrasonic imaging could be an efficient and accurate method to determine the state of gonads of mature sex-reversed rainbow trout females during spawning season. It could also be used as tool to the selection of individuals maturing earlier, selection of bisexual gonads or identification of differences in quality of neomales gametes. Although the duration of spermatogenesis in salmonids is quite long (Gjerde 1984; Munkittrick and Moccia 1987), the morphological size of testis undergoes wide seasonal variation (Billard 1986). Large variation in testis size has been attributed to the cyclical expansion and contraction of interstitial cells (Billard 1986), the loss of mass via ejaculation (Munkittrick and Moccia 1987), and the subsequent degeneration and loss of dead Leydig cells (Cauty and Loir 1995). Therefore, despite the long duration of testicular growth in male steelhead, changes in gonad size (i.e., a decrease) should be detectable with ultrasound imaging.
The results of starter diet administration (20 ppm OHA) obtained by Demska-Zakęś et al. (1999) confirmed used method as very effective for hormonal female sex reversal in rainbow trout. In group fed of diet supplemented 20 ppm OHA obtained 100 % males, while with addition 5 ppm MT, they noted 70 % males and 30 % bisexual individuals. Kuzminski and Dobosz (2010) applied 20 ppm OHA in the starter feed delivered to monosex female fry of rainbow trout for a period of 60 days resulted in a 96.6 % sex reversal into neomales, including 1.6 % bisexual individuals. A slightly higher dosage of 6 ppm was used for female sex reversal with MT resulted a 61.5 % share of sex-reversed neomales, including 1.8 % bisexual individuals. The rest of the fish were either mature females (36.7 %) or sterile individuals (1.8 %). Survival in the MT and OHA groups was similar at 38.0 and 40.7 %, and these small differences were caused by unknown factors. The GSI in sex-reversed females with MT and OHA was almost the same (3.9 %), similarly to our data. The GSI value has a direct influence on the total number of spermatozoa, which is very important for the reproductive cycle. The concentration of spermatozoa in the testis of rainbow trout males is 5.8 × 1010 g−1 of gonad, and the GSI is usually within the range of 6–8 % (Billard 1992). In our study, sperm concentration was very high—between 23.3 and 40.8 × 109 ml−1 spermatozoa (Table 1), but motility was very rewarding in all examined fish.
Throughout the reproductive season, the spermatozoa of normal male trout are stored in the spermatic duct (Ciereszko et al. 1996). In contrast, since spermatic ducts are absent in sex-reversed female rainbow trout, the spermatozoa remain within the testis (Tsumura et al. 1991). However, the absence of spermatic ducts in neomales means they can only be used once for reproduction (Bye and Lincoln 1986). When we use to collect sex-reversed females semen a syringe, the specimen can be exploited for the next few spawning seasons. Ultrasound technique has the potential to be a highly accurate, non-invasive diagnostic tool in this procedure. When operators are properly trained in use of the equipment and are knowledgeable about specimen anatomy, it is easy to examine position of gonadal lobes, particularly during spawning time. For example, in ultrasound studies conducted on adult rainbow trout, Reimers et al. (1987) were able to distinguish with 100 % accuracy the sex of specimens 5 months before spawning. Martin et al (1983) found that mature coho salmon could be sexed accurately with ultrasound 1 month before spawning. Ultrasound examination of striped bass Morone saxatilis allowed identification of the sex of fish with 100 % accuracy throughout the entire reproductive cycle (Blythe et al. 1994).
Normal males can be stripped repeatedly throughout the spawning season, although there are progressive changes in milt quality and sperm characteristics (Munkittrick and Moccia 1987). Sex-reversed females, however, present just one opportunity for milt extraction, making them an expensive resource. The use of ultrasound diagnostic offers new opportunities for the optimal control of environmental conditions, the selection of individuals maturing earlier, selection of bisexual specimens or identification of differences in quality of neomales gametes. Presented technique should be applied for verification of the effectiveness of fish sex inversion using hormonal therapy. Moreover, it can be helpful in determining the morphological structure of the gonads (paired–unpaired; symmetrical–asymmetrical testis), which will facilitate selection of sex-reversed females of rainbow trout during breeding procedures and consequently the production of an all-female population. Because of its high rate of speed and accuracy, ultrasonography may be considered for wider applications in fisheries management in order to protect the specimens with regular gonads and produce a good quality of semen. Furthermore, the results can be applied in selecting suitable fish for artificial propagation and monosex culture purposes. This method can also help to obtain advanced economic benefits of salmonid fish farms.
References
Billard R (1986) Spermatogenesis and spermatology of some teleost fish species. Reprod Nutr Dev 2:877–920
Billard R (1992) Reproduction in rainbow trout: sex differentiation, dynamics of gametogenesis, biology and preservation of gametes. Aquaculture 100:263–298
Blythe B, Helfrich LA, Beal WE, Boswarth B, Libey GS (1994) Determination of sex and maturational status of striped bass (Morone saxatilis) using ultrasonic imaging. Aquaculture 125:175–184
Bonar SA, Thomas GL, Pauley GB, Martin RW (1989) Use of ultrasonic images for rapid nonlethal determination of sex and maturity of Pacific herring. N Am J Fish Manage 9:364–366
Bye VJ, Lincoln RF (1986) Commercial method for the control of sexual maturation in rainbow trout (Salmo gairdneri R.). Aquaculture 57:299–309
Cauty C, Loir M (1995) The interstitial cells of the trout testis (Oncorhynchus mykiss): ultrastructural characterization and changes throughout the reproductive cycle. Tissue Cell 27(4):383–395
Ciereszko A, Liu L, Dabrowski K (1996) Effects of season and dietary ascorbic acid on some biochemical characteristics of rainbow trout Oncorhynchus mykiss semen. Fish Physiol Biochem 15:1–10
Demska-Zakęś K, Hliwa P, Matyjewicz P, Zakęś Z (1999) The effect of 17α-methyltestosterone and 11β-hydroxyandrostenedione on the development of reproductive system in rainbow trout (Oncorhynchus mykiss Walbaum). Arch Pol Fish 7:227–235
Dietrich GJ, Szpyrka A, Wojtczak M, Dobosz S, Goryczko K, Żakowski Ł, Ciereszko A (2005) Effects of UV irradiation and hydrogen peroxide on DNA fragmentation, motility and fertilizing ability of rainbow trout (Oncorhynchus mykiss) spermatozoa. Theriogenology 64:1809–1822
Donaldson EM (1996) Manipulation of reproduction in farmed fish. Anim Reprod Sci 42:381–392
Evans AF, Fitzpatrick MS, Siddens LK (2004) Use of ultrasound imaging and steroid concentrations to identify maturational status in adult steelhead. N Am J Fish Manage 24:967–978
Feist G, Yeoh CG, Fitzpatrick MS, Schreck CB (1995) The production of functional sex-reversed male rainbow trout with 17α-methylotestosterone and 11β-hydroxyandrostenedione. Aquaculture 131:145–152
Geffen AJ, Evans JP (2000) Sperm traits and fertilization success of male and sex-reversed female rainbow trout (Oncorhynchus mykiss). Aquaculture 182:61–72
Gjerde B (1984) Variation in semen production of farmed Atlantic salmon and rainbow trout. Aquaculture 40:109–114
Karlsen O, Holm JC (1994) Ultrasonography, a non-invasive method for sex determination in cod (Cadus morhua). J Fish Biol 44:965–971
Kuzminski H, Dobosz S (2010) Effect of sex reversal in rainbow trout (Oncorhynchus mykiss Walbaum) using 17α-methyltestosterone and 11β-hydroxyandrostenedione. Arch Pol Fish 18:45–49
Martin RM, Myers J, Sower SA, Phillips DJ, McAuley C (1983) Ultrasonic imaging—a potential tool for sex determination of live fish. N Am J Fish Manage 3:258–264
Mattson NS (1991) A new method to determination sex and gonad size in live fishes by using ultrasonography. J Fish Biol 39:673–677
Moghim M, Vajhi AR, Veshkini A, Masoudifard M (2002) Determination of sex and maturity in Acipenser stellatus by using ultrasonography. J Appl Ichthyol 18:325–328
Munkittrick KR, Moccia RD (1987) Seasonal changes in the quality of rainbow trout (Salmo gairdneri) semen: effects of a delay in stripping on spermatocrit, motility volume and seminal plasma constituents. Aquaculture 64:147–156
Nynca J, Ciereszko A (2009) Measurement of concentration and viability of brook trout (Salvelinus fontinalis) spermatozoa using computer-aided fluorescent microscopy. Aquaculture 292:256–258
Nynca J, Kuźmiński H, Dietrich GJ, Hliwa P, Dobosz S, Liszewska E, Karol H, Ciereszko A (2012) Biochemical and physiological characteristics of semen of sex-reversed females rainbow trout (Oncorhynchus mykiss, Walbaum). Theriogenology 77:174–183
Reimers E, Landmark P, Sorsdal T, Bohmer E, Solum T (1987) Determination of salmonids’ sex, maturation and size: an ultrasound and photocell approach. Aquac Mag 13:41–44
Robles V, Cabrita E, Cuñado S, Herráez MP (2003) Sperm cryopreservation of sex-reversed rainbow trout (Oncorhynchus mykiss): parameters that affect its ability for freezing. Aquaculture 224:203–212
Shields RJ, Davenport J, Young C, Smith PL (1993) Oocyte maturation and ovulation in the Atlantic halibut, Hippoglossus hippoglossus (L.), examined using ultrasonography. Aquac Res 24:181–186
Tsumura K, Blann VE, Lamont CA (1991) Progeny test of masculinized female rainbow trout having functional gonoducts. Prog Fish Cult 53:45–47
Acknowledgments
We thank MINDRAY Ltd. (distributor in Poland—Vetmedical, Poznan) for kindly providing USG equipment for the studies. We also thank Elzbieta Ziomek (Department of Ichthyology, University of Warmia and Mazury in Olsztyn) for technical assistance during analysis. This work was supported by a Project N N311 461639 from the Ministry of Sciences and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hliwa, P., Bah, M., Kuźmiński, H. et al. Ultrasound evaluation of the gonadal structure in sex-reversed rainbow trout females. Aquacult Int 22, 89–96 (2014). https://doi.org/10.1007/s10499-013-9646-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9646-5