Abstract
Purpose
Mucosal associated invariant T (MAIT) cells have properties of the innate and acquired immune systems. While the response to vigorous exercise has been established for most leukocytes, MAIT cells have not been investigated. Therefore, the purpose was to determine if MAIT cell lymphocytosis occurs with acute maximal aerobic exercise and if this response is influenced by exercise duration, cardiovascular fitness, or body composition.
Methods
Twenty healthy young males with moderate fitness levels performed an extended graded exercise test until volitional fatigue. Peripheral blood mononuclear cells were isolated from venous blood obtained prior and immediately after exercise and were labeled to identify specific T cell populations using flow cytometry.
Results
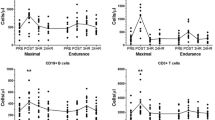
The percentage of MAIT cells relative to total T cells significantly increased from 3.0 to 3.8% and absolute MAIT cell counts increased by 2.2-fold following maximal exercise. MAIT cell subpopulation proportions were unchanged with exercise. Within cytotoxic T lymphocytes (CTL), MAIT cells consisted of 8% of these cells and this remained constant after exercise. MAIT cell counts and changes with exercise were not affected by body composition, VO2peak, or exercise duration.
Conclusions
Maximal exercise doubled MAIT cell numbers and showed preferential mobilization within total T cells but the response was not influenced by fitness levels, exercise duration, or body composition. These results suggest that acute exercise could be used to offset MAIT cell deficiencies observed with certain pathologies. MAIT cells also make up a substantial proportion of CTLs, which may have implications for cytotoxicity assays using these cells.




Similar content being viewed by others
Abbreviations
- 7-AAD:
-
7-Amino-actinomycin D
- CTL:
-
Cytotoxic T lymphocyte
- GXT:
-
Graded exercise test
- HbA1C:
-
Glycated hemoglobin
- MAIT cells:
-
Mucosal associated invariant T cell
- PBMC:
-
Peripheral blood mononuclear cell
- TCR:
-
T cell receptor
- VO2peak :
-
Peak oxygen uptake
- W:
-
Watts
References
Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B, Ramamurthy N, Zitzmann N, Barnes EJ, Thevanayagam J, Bhagwanani A, Leslie A, Oo YH, Kollnberger S, Bowness P, Drognitz O, Adams DH, Blum HE, Thimme R, Klenerman P (2010) Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA 107:3006–3011. doi:10.1073/pnas.0914839107
Bishop NC, Hayashida H, Clark M, Coombs C, Miller S, Stensel DJ (2014) Effect of acute and regular exercise on growth hormone secretagogue receptor-1a expression in human lymphocytes, T cell subpopulation and monocytes. Brain Behav Immun 39:172–179. doi:10.1016/j.bbi.2013.09.017
Campbell JP, Guy K, Cosgrove C, Florida-James GD, Simpson RJ (2008) Total lymphocyte CD8 expression is not a reliable marker of cytotoxic T-cell populations in human peripheral blood following an acute bout of high-intensity exercise. Brain Behav Immun 22:375–380. doi:10.1016/j.bbi.2007.09.001
Carolan E, Tobin LM, Mangan BA, Corrigan M, Gaoatswe G, Byrne G, Geoghegan J, Cody D, O’Connell J, Winter DC, Doherty DG, Lynch L, O’Shea D, Hogan AE (2015) Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J Immunol 194:5775–5780. doi:10.4049/jimmunol.1402945
Chen Z, Wang H, D’Souza C, Sun S, Kostenko L, Eckle SB, Meehan BS, Jackson DC, Strugnell RA, Cao H, Wang N, Fairlie DP, Liu L, Godfrey DI, Rossjohn J, McCluskey J, Corbett AJ (2017) Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol 10:58–68. doi:10.1038/mi.2016.39
Cosgrove C, Ussher JE, Rauch A, Gartner K, Kurioka A, Huhn MH, Adelmann K, Kang YH, Fergusson JR, Simmonds P, Goulder P, Hansen TH, Fox J, Gunthard HF, Khanna N, Powrie F, Steel A, Gazzard B, Phillips RE, Frater J, Uhlig H, Klenerman P (2013) Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood 121:951–961. doi:10.1182/blood-2012-06-436436
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O (2011) Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117:1250–1259. doi:10.1182/blood-2010-08-303339
Fergusson JR, Huhn MH, Swadling L, Walker LJ, Kurioka A, Llibre A, Bertoletti A, Hollander G, Newell EW, Davis MM, Sverremark-Ekstrom E, Powrie F, Capone S, Folgori A, Barnes E, Willberg CB, Ussher JE, Klenerman P (2016) CD161(int)CD8+ T cells: a novel population of highly functional, memory CD8+ T cells enriched within the gut. Mucosal Immunol 9:401–413. doi:10.1038/mi.2015.69
Fernandez CS, Amarasena T, Kelleher AD, Rossjohn J, McCluskey J, Godfrey DI, Kent SJ (2015) MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol Cell Biol 93:177–188. doi:10.1038/icb.2014.91
Fyfe JJ, Bartlett JD, Hanson ED, Stepto NK, Bishop DJ (2016) Endurance training intensity does not mediate interference to maximal lower-body strength gain during short-term concurrent training. Front Physiol 7:487. doi:10.3389/fphys.2016.00487
Gabriel H, Schwarz L, Born P, Kindermann W (1992) Differential mobilization of leucocyte and lymphocyte subpopulations into the circulation during endurance exercise. Eur J Appl Physiol Occup Physiol 65:529–534
Gannon GA, Rhind SG, Shek PN, Shephard RJ (2001) Differential cell adhesion molecule expression and lymphocyte mobilisation during prolonged aerobic exercise. Eur J Appl Physiol 84:272–282. doi:10.1007/s004210000374
Gherardin NA, Keller AN, Woolley RE, Le Nours J, Ritchie DS, Neeson PJ, Birkinshaw RW, Eckle SB, Waddington JN, Liu L, Fairlie DP, Uldrich AP, Pellicci DG, McCluskey J, Godfrey DI, Rossjohn J (2016) Diversity of T cells restricted by the MHC Class I-related molecule MR1 facilitates differential antigen recognition. Immunity 44:32–45. doi:10.1016/j.immuni.2015.12.005
Gleeson M, Bishop NC (2005) The T cell and NK cell immune response to exercise. Ann Transplant 10:43–48
Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB (2015) The burgeoning family of unconventional T cells. Nat Immunol 16:1114–1123. doi:10.1038/ni.3298
Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM (2010) Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 8:e1000407. doi:10.1371/journal.pbio.1000407
Gold MC, Eid T, Smyk-Pearson S, Eberling Y, Swarbrick GM, Langley SM, Streeter PR, Lewinsohn DA, Lewinsohn DM (2013) Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol 6:35–44. doi:10.1038/mi.2012.45
Gold MC, McLaren JE, Reistetter JA, Smyk-Pearson S, Ladell K, Swarbrick GM, Yu YY, Hansen TH, Lund O, Nielsen M, Gerritsen B, Kesmir C, Miles JJ, Lewinsohn DA, Price DA, Lewinsohn DM (2014) MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. J Exp Med 211:1601–1610. doi:10.1084/jem.20140507
Gold MC, Napier RJ, Lewinsohn DM (2015) MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev 264:154–166. doi:10.1111/imr.12271
Hansen JB, Wilsgard L, Osterud B (1991) Biphasic changes in leukocytes induced by strenuous exercise. Eur J Appl Physiol Occup Physiol 62:157–161
Hiejima E, Kawai T, Nakase H, Tsuruyama T, Morimoto T, Yasumi T, Taga T, Kanegane H, Hori M, Ohmori K, Higuchi T, Matsuura M, Yoshino T, Ikeuchi H, Kawada K, Sakai Y, Kitazume MT, Hisamatsu T, Chiba T, Nishikomori R, Heike T (2015) Reduced numbers and proapoptotic features of mucosal-associated invariant T cells as a characteristic finding in patients with inflammatory bowel disease. Inflamm Bowel Dis 21:1529–1540. doi:10.1097/MIB.0000000000000397
Hinks TS, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, Lum PY, Smith CG, Ward JA, Howarth PH, Walls AF, Gadola SD, Djukanovic R (2015) Innate and adaptive T cells in asthmatic patients: relationship to severity and disease mechanisms. J Allergy Clin Immunol 136:323–333. doi:10.1016/j.jaci.2015.01.014
Hong S, Johnson TA, Farag NH, Guy HJ, Matthews SC, Ziegler MG, Mills MJ (2005) Attenuation of T-lymphocyte demargination and adhesion molecule expression in response to moderate exercise in physically fit individuals. J Appl Physiol (1985) 98:1057–1063. doi:10.1152/japplphysiol.00233.2004
Kendall A, Hoffman-Goetz L, Houston M, MacNeil B, Arumugam Y (1990) Exercise and blood lymphocyte subset responses: intensity, duration, and subject fitness effects. J Appl Physiol (1985) 69:251–260
Koay HF, Gherardin NA, Enders A, Loh L, Mackay LK, Almeida CF, Russ BE, Nold-Petry CA, Nold MF, Bedoui S, Chen Z, Corbett AJ, Eckle SB, Meehan B, d’Udekem Y, Konstantinov IE, Lappas M, Liu L, Goodnow CC, Fairlie DP, Rossjohn J, Chong MM, Kedzierska K, Berzins SP, Belz GT, McCluskey J, Uldrich AP, Godfrey DI, Pellicci DG (2016) A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol 17:1300–1311. doi:10.1038/ni.3565
Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, Kang YH, Walker LJ, Hansen TH, Willberg CB, Klenerman P (2015) MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol 8:429–440. doi:10.1038/mi.2014.81
Loh L, Wang Z, Sant S, Koutsakos M, Jegaskanda S, Corbett AJ, Liu L, Fairlie DP, Crowe J, Rossjohn J, Xu J, Doherty PC, McCluskey J, Kedzierska K (2016) Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc Natl Acad Sci USA 113:10133–10138. doi:10.1073/pnas.1610750113
Magalhaes I, Pingris K, Poitou C, Bessoles S, Venteclef N, Kiaf B, Beaudoin L, Da Silva J, Allatif O, Rossjohn J, Kjer-Nielsen L, McCluskey J, Ledoux S, Genser L, Torcivia A, Soudais C, Lantz O, Boitard C, Aron-Wisnewsky J, Larger E, Clement K, Lehuen A (2015) Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest 125:1752–1762. doi:10.1172/JCI78941
Maillard F, Rousset S, Pereira B, Traore A, Del Amaze PDP, Boirie Y, Duclos M, Boisseau N (2016) High-intensity interval training reduces abdominal fat mass in postmenopausal women with type 2 diabetes. Diabetes Metab 42:433–441. doi:10.1016/j.diabet.2016.07.031
Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, Cherif S, Vera G, Latour S, Soudais C, Lantz O (2009a) Stepwise development of MAIT cells in mouse and human. PLoS Biol 7:e54. doi:10.1371/journal.pbio.1000054
Martin SA, Pence BD, Woods JA (2009b) Exercise and respiratory tract viral infections. Exerc Sport Sci Rev 37:157–164. doi:10.1097/JES.0b013e3181b7b57b
Nieman DC (1994) Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc 26:128–139
Nieman DC, Henson DA, Johnson R, Lebeck L, Davis JM, Nehlsen-Cannarella SL (1992) Effects of brief, heavy exertion on circulating lymphocyte subpopulations and proliferative response. Med Sci Sports Exerc 24:1339–1345
Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I (2007) A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond) 31:1786–1797. doi:10.1038/sj.ijo.0803683
Rossiter HB, Kowalchuk JM, Whipp BJ (2006) A test to establish maximum O2 uptake despite no plateau in the O2 uptake response to ramp incremental exercise. J Appl Physiol (1985) 100:764–770. doi:10.1152/japplphysiol.00932.2005
Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O, Dupas JL, Treiner E (2014) Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol 176:266–274. doi:10.1111/cei.12277
Simpson RJ, Cosgrove C, Ingram LA, Florida-James GD, Whyte GP, Pircher H, Guy K (2008) Senescent T-lymphocytes are mobilised into the peripheral blood compartment in young and older humans after exhaustive exercise. Brain Behav Immun 22:544–551. doi:10.1016/j.bbi.2007.11.002
Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC, Kunz H (2012) Exercise and the aging immune system. Ageing Res Rev 11:404–420. doi:10.1016/j.arr.2012.03.003
Takahashi T, Dejbakhsh-Jones S, Strober S (2006) Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol 176:211–216
Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O (1999) An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med 189:1907–1921
Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422:164–169. doi:10.1038/nature01433
Ussher JE, Klenerman P, Willberg CB (2014) Mucosal-associated invariant T-cells: new players in anti-bacterial immunity Front Immunol 5:450. doi:10.3389/fimmu.2014.00450
Walker LJ, Kang YH, Smith MO, Tharmalingham H, Ramamurthy N, Fleming VM, Sahgal N, Leslie A, Oo Y, Geremia A, Scriba TJ, Hanekom WA, Lauer GM, Lantz O, Adams DH, Powrie F, Barnes E, Klenerman P (2012) Human MAIT and CD8alphaalpha cells develop from a pool of type-17 precommitted CD8+ T cells. Blood 119:422–433. doi:10.1182/blood-2011-05-353789
Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P (2011) Position statement. Part one: immune function and exercise. Exerc Immunol Rev 17:6–63
Willis LH, Slentz CA, Bateman LA, Shields AT, Piner LW, Bales CW, Houmard JA, Kraus WE (2012) Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (1985) 113:1831–1837. doi:10.1152/japplphysiol.01370.2011
Zhang N, Bevan MJ (2011) CD8(+) T cells: foot soldiers of the immune system. Immunity 35:161–168. doi:10.1016/j.immuni.2011.07.010
Acknowledgements
The authors would like to thank the volunteers for their involvement in this study and Ms. Shadney Que and Ms. Chantelle Blythe for their assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the Collaborative Research Network from the Department of Industry, Innovation, and Science of Australia.
Additional information
Communicated by Fabio Fischetti.
Electronic supplementary material
Below is the link to the electronic supplementary material.
421_2017_3704_MOESM1_ESM.pdf
Supplemental Fig. 1 Immunofluorescence gating strategy for the identification of the proportion of (relative to the parent population) and the absolute numbers of MAIT cells with acute exercise. A) Gating of single PBMCs, B) the inclusion of CD45+ cells, C) identifying lymphocytes, and D) gating for T (CD3+) and the E) CD4+ and CD8+ T cell subpopulations. Subsequently, cells that express Vα7.2 and CD161 were determined from F) cytotoxic T lymphocytes CDLs (CD8+) and G) CD4. H) From the total T cell population (panel D), Vα7.2+CD161+ MAIT cells were identified and the G) MAIT cell subpopulations of CD4+ and CD8+ (PDF 145 kb)
Rights and permissions
About this article
Cite this article
Hanson, E.D., Danson, E., Nguyen-Robertson, C.V. et al. Maximal exercise increases mucosal associated invariant T cell frequency and number in healthy young men. Eur J Appl Physiol 117, 2159–2169 (2017). https://doi.org/10.1007/s00421-017-3704-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3704-z