Abstract
Purpose
To determine the frequency of obstetrical adverse events and clinical outcome in infants following antenatal hyperimmune globulin (HIG) treatment for primary cytomegalovirus (CMV) infection in pregnancy.
Methods
Data from 50 women including three twin pregnancies were retrospectively evaluated. Primary infection was defined by seroconversion or the presence of CMV-specific IgM and low IgG avidity. All women received two or more infusions of HIG (200 U/kg). Congenital CMV (cCMV) infection was diagnosed by detection of CMV in amniotic fluid and/or neonatal urine. We compared gestational age (GA) at birth, head circumference (HC) and birth weight (BW) of infants in our study cohort with those of live-born infants delivered in our clinic between 2015 and 2016.
Results
Median gestational age at time of maternal CMV diagnosis was 13 weeks. One-hundred-forty-one maternal HIG doses were given. No HIG-related severe adverse reactions occurred. Preterm birth rate was 4.2% (2/47) in singleton pregnancies. None of the neonates had birth weight or head circumference < 3rd percentile (< 3P) for gestational age. There was no statistically significant difference regarding GA, BW and HC between our study cohort and the total population of live-born infants. The frequency of CMV-related sequelae in infants with cCMV infection was 10.5% (2/19) (one with bilateral hearing loss and one with mild motoric delay), both cases following first trimester maternal infection.
Conclusion
Antenatal HIG treatment was well tolerated and not associated with prematurity or decreased birth weight. HIG application might have a favorable effect on the clinical course of congenital CMV infection.
Similar content being viewed by others
Introduction
Congenital cytomegalovirus (cCMV) infection is the leading infectious cause of childhood disability in developed countries. It is estimated that 10–15% of infants with cCMV infection are symptomatic at birth and 40–60% of these children develop long-term sequelae including hearing loss, cerebral palsy, seizures, neurodevelopmental delay and loss of vision [1].
Materno-fetal transmission of CMV may occur throughout pregnancy. The intrauterine transmission rate after primary infection increases from approximately 30% in early pregnancy to 70% in the third trimester. Primary infection around conception and in the first trimester bears the highest risk for congenital CMV disease [2,3,4]. However, severe cases of congenital infection can also occur following a maternal CMV recurrence. CMV-derived damage of the fetal brain is caused by cell destruction, vasculitis and dysfunction in brain development. In addition, it has been suggested that CMV infection can lead to placental cell dysfunction with consecutive fetal injury [5, 6].
No licensed drugs are available for prevention or therapy of fetal CMV infection. The antiviral valacyclovir has been suggested as a possible treatment for infected fetuses with mild ultrasound abnormalities [7]. A recent review on prophylaxis and treatment of congenital CMV infection showed a trend towards efficacy of CMV hyperimmune globulin (HIG). However, available data do not allow a final conclusion on HIG utility [8]. In addition, the only randomized study covering this topic raised concerns that HIG exposure in pregnancy is associated with obstetrical adverse events [9]. This observation, though, was questioned by the results of another study [10].
In our clinic, women with primary CMV infection are counseled about possible consequences of congenital CMV infection, the utility of targeted ultrasound controls, feasibility of invasive prenatal diagnosis and availability of antenatal off-label treatment options based on published data.
The aim of this study was to assess the tolerability and possible side effects of HIG in pregnant women with primary CMV infection who opted for such a treatment. Secondarily, we evaluated the efficacy of HIG in preventing intrauterine CMV infection or cCMV-associated morbidity.
Materials and methods
Between 2007 and 2016, 51 women started treatment with CMV HIG in our prenatal medicine unit. The women were referred to our tertiary center because of suspected primary CMV infection. None of them was referred because of abnormal ultrasound (US) findings.
At first presentation, a detailed medical and obstetrical history was obtained. Patients were counseled on possible consequences of CMV infection (e.g., risk of intrauterine transmission and clinical sequelae) and serological CMV diagnosis was repeated to confirm existing results and/or to better determine the time of maternal infection. Acute primary CMV infection was defined by IgG seroconversion or the presence of CMV-specific IgM and low IgG avidity.
In case of a confirmed infection, targeted ultrasound examinations were performed every 2–3 weeks till delivery by the same experienced examiner. Ultrasound features compatible with congenital CMV disease (cerebral abnormality: ventriculomegaly, cerebral calcification, lissencephaly, abnormal corpus callosum, periventricular cysts; extracerebral abnormality: hydrops, hepatomegaly, skin edema, hyperechogenic bowel), other major or minor anomalies, fetal growth and estimated fetal weight (EFW), amniotic fluid (AF) volume, placental appearance and cervix length were assessed.
Each patient was advised about feasibility of amniocentesis for prenatal diagnosis of fetal infection. Invasive prenatal diagnosis was only performed after 20 weeks gestation (WG) and at least 8 weeks after maternal infection. Patients with a positive prenatal diagnosis (detection of CMV in AF by polymerase chain reaction-PCR- or viral culture) were also offered cordocentesis to determine fetal blood parameters (e.g., platelet count, viral load) that could assist in the prediction of clinical outcome at birth. Women who opted for treatment received at least two weight-based intravenous doses of CMV HIG (200 U/kg) every 2–3 weeks.
In women with a positive prenatal diagnosis who opted for cordocentesis off-label intraumbilical administration of CMV-HIG during fetal blood sampling was also discussed.
Most of the women (73%) delivered in our clinic, and the presence of congenital infection was assessed by detection of CMV in neonatal urine using PCR and viral culture. Information on obstetrical and neonatal outcome (e.g., gestational age at delivery, delivery mode, birth weight, head circumference, clinical and laboratory findings) and clinical follow-up was obtained from hospital charts (including electronic records), telephone interviews or a written questionnaire sent to the families. Congenital CMV disease was defined according to Rawlinson et al. [11].
We compared gestational age (GA) at birth, head circumference (HC) and birth weight (BW) of infants in our study cohort with those of live-born infants delivered in our clinic between 2015 and 2016. Demographic and obstetrical data of the latter group were retrospectively extracted from the perinatal data base. Comparisons were done with a Mann–Whitney test using MedCalcR software version 11.4.1.0.
Approval of the present study was obtained by the local ethic committee of our perinatology tertiary centre. Women who opted for CMV-HIG-application gave their written informed consent to be treated off-label and were prospectively followed up in our hospital.
Results
One of the 51 women experienced a short, self-limited episode of nausea and tachycardia a few minutes after the first HIG infusion was started (at a rate of 6 ml/h). The episode was not accompanied by rash, dyspnea, edema, vomiting or hypotension. The woman was very anxious about an allergic reaction already before start of treatment and she decided to discontinue HIG. This patient was excluded from the analysis.
Fifty women completed HIG prophylaxis or treatment. Three of the 50 pregnancies were dichorionic diamniotic twins, so that we could enroll 53 infants in the study. In ten cases, it was the first pregnancy. Two women had pre-existing diseases (HIV infection and multiple sclerosis). Maternal characteristics are presented in Table 1. Primary CMV infection was diagnosed in the first trimester in 20 women (including three twin pregnancies), in the second trimester in 27 women and in the third trimester in three women. HIG prophylaxis or treatment was started in the first, second and third trimester in 20, 23 and 7 women, respectively. A total of 141 maternal doses were given. Each woman received at least two doses (range 2–5). Median number of doses per patient was three. HIG was well tolerated in all but three applications (2.1%). These patients reported flu-like symptoms within 24 h of HIG infusion. Pregnancy-related diseases developed in two women (gestational diabetes and intrahepatic cholestasis).
At serial scans we found major fetal anomalies in 2 fetuses (7%) and minor fetal anomalies in 6 fetuses (11%) (Table 2). None of the fetuses was growth restricted (EFW < 3 P). Fetal Magnetic Resonance Imaging (MRI) was carried out in six cases between 25 and 30 weeks of gestation and always gave a normal result.
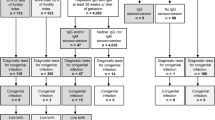
Twenty-five women underwent amniocentesis to rule out a fetal infection (CMV DNA -real time PCR and amniotic fluid culture). Congenital CMV infection was excluded during prenatal diagnosis and at birth in 12 fetuses/infants. In one case, prenatal diagnosis was negative but the newborn presented with asymptomatic cCMV infection. Twelve women had a positive amniotic fluid and confirmed cCMV at birth. In four of them amniotic fluid was positive before start of HIG treatment. One fetus was concomitantly affected by Down syndrome. Cordocentesis was done in eight fetuses with a positive AF sample and six fetuses received an intraumbilical HIG infusion (800–1000 U/kg). None of the women opted for a termination of pregnancy (TOP). No intrauterine fetal death occurred (IUFD). Seventy-two percent of the women delivered spontaneously. Preterm birth occurred in 4.2% (2/47) of singleton and 8% (4/50) of all pregnancies (including 3 pairs of twins). Birth before 32 GW was observed in 2.0% (1/50) of cases.
Overall, cCMV infection was diagnosed during prenatal diagnosis or at birth in 19 of 53 fetuses. Two of the 19 CMV-positive infants (10.5%) were symptomatic at birth (Table 3). One of the symptomatic infants also had trisomy 21. Four infants showed signs of lenticulostriate vasculopathy (LSV) on brain ultrasound. However, three of them were otherwise normal. In one twin pregnancy we observed a non-concordant intrauterine transmission. Excluding four fetuses with a positive prenatal diagnosis before starting therapy, the rate of intrauterine transmission in women with prophylactic HIG was 30.6% (15/49). The rate of transmission according to gestational age at diagnosis was 39.1% (9/23) in the first trimester, 26% (6/23) in the second trimester and 0% (0/3) in the third trimester. In our study cohort, median GA at delivery was 39.3 weeks (IQR 38.3; 40.4), median BW was 3200 g (IQR 2947; 3611) and median HC was 35.0 cm (IQR 33.5; 35.0). These parameters did not differ between infants with and without cCMV infection (Table 4). None of the neonates had birthweight or head circumference < 3rd percentile (< 3P) for gestational age. The frequency of CMV-related sequelae in infants with cCMV infection was 10.5% (2/19) (one with bilateral hearing loss and one with mild motoric delay), both cases following first trimester maternal infection.
In 2015 and 2016, a total of 6,376 women delivered in our clinic. Median maternal age was 32 years (IQR: 29; 36). Two hundred and ninety-eight (4.67%) women were pregnant with multiples. Information on GA at birth, HC and BW was available for 6,505 newborns. Median GA was 39.3 weeks (IQR 38.1; 40.4), median BW was 3285 g (2900; 3 630) and median HC was 34.5 cm (IQR 33.5; 35.5). There was no statistically significant difference regarding GA, BW and HC between our study cohort and the total population of live-born infants (Fig. 1).
Discussion
HIG has been suggested for the treatment of primary CMV infection in pregnancy to reduce the risk of intrauterine viral transmission and cCMV-derived morbidity [12, 13]. A recently published review indicated a trend towards efficacy of HIG, however definitive evidence of benefit for treatment is lacking [8]. Remarkably, Revello et al. [9] found in their randomized study a higher rate of preterm deliveries following antenatal HIG exposure compared to unexposed pregnancies [15% (7/48) vs. 2% (1/47)]. This observation raised concerns regarding the use of CMV-HIG in pregnant women.
The primary aim of our analysis was to assess tolerability of CMV-HIG during pregnancy and possible complications regarding pregnancy outcome. We observed no serious adverse events following HIG infusion. The overall rate of minor adverse events of 2.1% was similar to the rate of 1.7% reported by Buxmann et al. [14]. In our study cohort, preterm birth < 37 GW occurred in 4.2% (2/47) of singleton and 8% (4/50) of all pregnancies (including 3 pairs of twins). Birth < 32 GW was observed in 2.0% (1/50) of women. These figures are comparable with those reported in the perinatal survey for the German population in general [birth < 37 GW: 8.6% (66,851/773,338); birth < 32 GW: 1.5% (11,535/773,338)] [https://iqtig.org/downloads/ergebnisse/bundesauswertung/2016/indirekte_verfahren/QSKH_16n1-GEBH_2016_BUAW_V02_2017-07-12.pdf Accessed 18 January 2018].
During the study period most pregnant women with a confirmed primary CMV infection who decided to continue their pregnancy opted for an off-label treatment with HIG. A control group of primarily CMV infected women without HIG exposure was not available. Therefore, we compared the findings on GA at birth as well as HC and BW of infants in the study cohort with those of all live-born infants delivered in our clinic between 2015 and 2016. The comparison between these groups gave no evidence for a higher risk of prematurity (< 37 GW), low birth weight (< 10 P) or microcephaly (head circumference < 3P) following CMV-HIG exposure in pregnancy. Our observations are in agreement with those of Nigro and colleagues who did not find an association between HIG administration and prematurity or low birth weight in a cohort of 358 pregnant women, 164 of whom received HIG infusions [10]. Immune globulin is routinely used as post-exposure prophylaxis in pregnant women to prevent specific infections or ameliorate the clinical course of disease (e.g., chickenpox, measles). Moreover, immune globulin is given during gestation in other conditions such as autoimmune thrombocytopenia and immune deficiency syndromes. So far, the use of immune globulin in pregnancy has not been associated with obstetrical complications. Although this issue has not been addressed in larger controlled studies, we think that the available evidence does not indicate that preterm birth is a hyperimmune globulin-related adverse event.
Secondarily, we evaluated the rate of intrauterine viral transmission and clinical outcome in infants with cCMV. Overall, we observed no significant reduction in the rate of maternal–fetal transmission compared to published data. However, there was a trend towards a lower transmission rate for women diagnosed and treated in the second trimester (26 vs. 40.1%) [4]. In our cases median time interval between diagnosis and start of treatment was about 2 weeks and time between HIG infusions was 2–3 weeks. A more rigorous treatment schedule as suggested by Kagan and colleagues [i.e., start of HIG applications within 1 week of diagnosis and a strict 2 weekly dosing intervals of HIG (200 U/kg)] might be more effective in preventing intrauterine CMV transmission (Kagan et al. abstract 6th international congenital CMV conference, 2017, unpublished data).
We diagnosed cCMV infection in 19 newborns, 12 of whom already had a positive prenatal diagnosis. Maternal infection was diagnosed during the first trimester and second trimester in 14 and 5 cases, respectively. It has been shown that maternal infection around conception and the first trimester is a risk factor for symptomatic fetal infection, but infections in the second trimester may also result in congenital CMV disease [2,3,4, 15]. CMV-related US abnormalities have been described in the literature following periconception/first trimester and second trimester infections in 19–51 and 4–14%, respectively [2, 3, 15]. In our cohort, mild US abnormalities occurred in two fetuses (14%) whose mothers were diagnosed in the first trimester and in one fetus (25%) following second trimester diagnosis. The latter one also had trisomy 21. None of the women in our study opted for TOP and no IUFD occurred. Gestational age at birth, head circumference and birth weight did not differ between infants with and without cCMV infection. Importantly, we did not see a case of severe central nervous system (CNS) damage or cytomegalic inclusion disease. The most severe symptoms at delivery were seen in the newborn with cCMV and trisomy 21. And it remains unclear how much CMV contributed to the clinical findings. The most common abnormalities at birth were signs of lenticulostriate vasculopathy (LSV) on brain ultrasound (n = 4). We did not consider LSV as a brain marker of symptomatic cCMV infection because it can be seen in many other conditions and also in disease-free children [16, 17]. The frequency of sequelae in our cohort following first trimester maternal infection was 14% (2/14). This is lower than the rate of 32% (11/34) for CNS sequelae in first trimester infections (including hearing loss, abnormal neuropsychological findings, cerebral palsy, seizures, chorioretinitis) reported by Pass et al. [18]. However, our outcome data are limited by the fact that only 53% of infants with cCMV infection had follow-up more than 2 years (Table 3).
Conclusions
CMV-HIG was well tolerated in pregnant women and not associated with obstetric complications. HIG application might have a favorable effect on the clinical course of congenital CMV infection. Limitations of our study are the small number of cases and the retrospective design, but its force is the homogeneity of the available data since all patients were managed in one centre. Since the risk of severe cCMV disease is relatively small even following first trimester maternal infection a larger sample size would be necessary to detect a true benefit of HIG treatment.
References
Dollard SC, Grosse SD, Ross DS (2007) New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 17(5):355–363
Enders G, Daiminger A, Bäder U, Exler S, Enders M (2011) Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol 52(3):244–246
Picone O, Vauloup-Fellous C, Cordier AG, Guitton S, Senat MV, Fuchs F, Ayoubi JM, Grangeot Keros L, Benachi A (2013) A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenat Diagn 33(8):751–758
Bodeus M, Kabamba-Mukadi B, Zech F et al (2009) Human cytomegalovirus in utero transmission: follow-up of 524 maternal seroconversions. J Clin Virol 47:201–202
Born E (1955) Über frühkindliche Hirnschädigung bei Cytomegalie und ihre Abgrenzung gegenüber Toxoplasmose. Archiv für Psychiatrie und Zeitschrift Neurologie 193:557–568
Weisblum Y, Panet A, Haimov-Kochman R, Wolf DG (2014) Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin Immunopathol 36(6):615–625
Leruez-Ville M, Ghout I, Bussières L, Stirnemann J, Magny JF, Couderc S, Salomon LJ, Guilleminot T, Aegerter P, Benoist G, Winer N, Picone O, Jacquemard F, Ville Y (2016) In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol 215(4):462.e1–462.e10
Rawlinson WD, Hamilton ST, van Zuylen WJ (2016) Update on treatment of cytomegalovirus infection in pregnancy and of the newborn with congenital cytomegalovirus. Curr Opin Infect Dis 29(6):615–624
Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G, CHIP Study Group (2014) A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370(14):1316–1326
Nigro G, Capretti I, Manganello AM, Best AM, Adler SP (2015) Primary maternal cytomegalovirus infections during pregnancy: association of CMV hyperimmune globulin with gestational age at birth and birth weight. J Matern Fetal Neonatal Med 28(2):168–171
Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, Daly K, Doutré S, Gibson L, Giles ML, Greenlee J, Hamilton ST, Harrison GJ, Hui L, Jones CA, Palasanthiran P, Schleiss MR, Shand AW, van Zuylen WJ (2017) Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 17(6):e177–e188
Nigro G, Adler SP, La Torre R, Best AM (2005) Congenital Cytomegalovirus Collaborating Group: passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353(13):1350–1362
Hamprecht K, Kagan KO, Goelz R (2014) Hyperimmune globulin to prevent congenital CMV infection. N Engl J Med 370(26):2543
Buxmann H, Stackelberg OM, Schlößer RL, Enders G, Gonser M, Meyer-Wittkopf M, Hamprecht K, Enders M (2012) Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: a retrospective analysis. J Perinat Med 40(4):439–446
Feldman B, Yinon Y, Tepperberg Oikawa M, Yoeli R, Schiff E, Lipitz S (2011) Pregestational, periconceptional, and gestational primary maternal cytomegalovirus infection: prenatal diagnosis in 508 pregnancies. Am J Obstet Gynecol 205(4):342.e1-6
Giannattasio A, Di Costanzo P, Milite P, De Martino D, Capone E, Romano A, Bravaccio C, Capasso L, Raimondi F (2017) Is lenticulostriated vasculopathy an unfavorable prognostic finding in infants with congenital cytomegalovirus infection? J Clin Virol 91:31–35
Bilavsky E, Schwarz M, Pardo J, Attias J, Levy I, Haimi-Cohen Y, Amir J (2015) Lenticulostriated vasculopathy is a high-risk marker for hearing loss in congenital cytomegalovirus infections. Acta Paediatr 104(9):e388–e394
Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S (2006) Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol 35(2):216–220
Funding
None.
Author information
Authors and Affiliations
Contributions
DC: conception and design of the study, acquisition of data and data analysis, manuscript writing and editing. PN: acquisition of the data. MV: acquisition of data, manuscript writing. AL: data management. UK: manuscript writing. ME: acquisition of the data and data analysis, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the local ethic committee of the perinatology tertiary centre Olgahospital/Frauenklinik, Stuttgart. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All women who opted for HIG treatment gave informed written consent. The women clearly understood that at present HIG treatment is off-label and without evidence of efficacy.
Rights and permissions
About this article
Cite this article
Chiaie, L.D., Neuberger, P., Vochem, M. et al. No evidence of obstetrical adverse events after hyperimmune globulin application for primary cytomegalovirus infection in pregnancy: experience from a single centre. Arch Gynecol Obstet 297, 1389–1395 (2018). https://doi.org/10.1007/s00404-018-4703-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4703-y