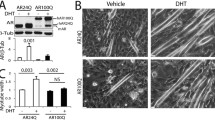
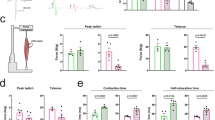
Abstract
Spinal and bulbar muscular atrophy (SBMA) is an inherited neuromuscular disease caused by expansion of a polyglutamine (polyQ) tract in the androgen receptor (AR). SBMA is triggered by the interaction between polyQ-AR and its natural ligands, testosterone and dihydrotestosterone (DHT). SBMA is characterized by the loss of lower motor neurons and skeletal muscle fasciculations, weakness, and atrophy. To test the hypothesis that the interaction between polyQ-AR and androgens exerts cell-autonomous toxicity in skeletal muscle, we characterized the process of myogenesis and polyQ-AR expression in DHT-treated satellite cells obtained from SBMA patients and age-matched healthy control subjects. Treatment with androgens increased the size and number of myonuclei in myotubes from control subjects, but not from SBMA patients. Myotubes from SBMA patients had a reduced number of nuclei, suggesting impaired myotube fusion and altered contractile structures. The lack of anabolic effects of androgens on myotubes from SBMA patients was not due to defects in myoblast proliferation, differentiation or apoptosis. DHT treatment of myotubes from SBMA patients increased nuclear accumulation of polyQ-AR and decreased the expression of interleukin-4 (IL-4) when compared to myotubes from control subjects. Following DHT treatment, exposure of myotubes from SBMA patients with IL-4 treatment rescued myonuclear number and size to control levels. This supports the hypothesis that androgens alter the fusion process in SBMA myogenesis. In conclusion, these results provide evidence of an androgen-dependent impairment of myogenesis in SBMA that could contribute to disease pathogenesis.







Similar content being viewed by others
References
Abmayr SM, Pavlath GK (2012) Myoblast fusion: lessons from flies and mice. Development 139:641–656. doi:10.1242/dev.068353;10.1242/dev.068353
Adachi H, Katsuno M, Minamiyama M, Waza M, Sang C, Nakagomi Y, Kobayashi Y, Tanaka F, Doyu M, Inukai A, Yoshida M, Hashizume Y, Sobue G (2005) Widespread nuclear and cytoplasmic accumulation of mutant androgen receptor in SBMA patients. Brain 128:659–670. doi:10.1093/brain/awh381
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15:6541–6551
Amato AA, Prior TW, Barohn RJ, Snyder P, Papp A, Mendell JR (1993) Kennedy’s disease: a clinicopathologic correlation with mutations in the androgen receptor gene. Neurology 43:791–794
Arnold AS, Gueye M, Guettier-Sigrist S, Courdier-Fruh I, Coupin G, Poindron P, Gies JP (2004) Reduced expression of nicotinic AChRs in myotubes from spinal muscular atrophy I patients. Lab Invest 84:1271–1278. doi:10.1038/labinvest.3700163
Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R (1997) Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 82:407–413
Brodsky IG, Balagopal P, Nair KS (1996) Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men–a clinical research center study. J Clin Endocrinol Metab 81:3469–3475
Chevalier-Larsen ES, O’Brien CJ, Wang H, Jenkins SC, Holder L, Lieberman AP, Merry DE (2004) Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci 24:4778–4786. doi:10.1523/JNEUROSCI.0808-04.2004
Doumit ME, Cook DR, Merkel RA (1996) Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology 137:1385–1394
Glass D, Roubenoff R (2010) Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci 1211:25–36. doi:10.1111/j.1749-6632.2010.05809.x
Guidetti D, Vescovini E, Motti L, Ghidoni E, Gemignani F, Marbini A, Patrosso MC, Ferlini A, Solime F (1996) X-linked bulbar and spinal muscular atrophy, or Kennedy disease: clinical, neurophysiological, neuropathological, neuropsychological and molecular study of a large family. J Neurol Sci 135:140–148
Harding AE, Thomas PK, Baraitser M, Bradbury PG, Morgan-Hughes JA, Ponsford JR (1982) X-linked recessive bulbospinal neuronopathy: a report of ten cases. J Neurol Neurosurg Psychiatry 45:1012–1019
Horsley V, Jansen KM, Mills ST, Pavlath GK (2003) IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113:483–494
Kadi F (2008) Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol 154:522–528. doi:10.1038/bjp.2008.118
Kang JS, Krauss RS (2010) Muscle stem cells in developmental and regenerative myogenesis. Curr Opin Clin Nutr Metab Care 13:243–248. doi:10.1097/MCO.0b013e328336ea98
Katsuno M, Adachi H, Kume A, Li M, Nakagomi Y, Niwa H, Sang C, Kobayashi Y, Doyu M, Sobue G (2002) Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35:843–854
Kemppainen JA, Lane MV, Sar M, Wilson EM (1992) Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. J Biol Chem 267:968–974
Kennedy WR, Alter M, Sung JH (1968) Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology 18:671–680
La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352:77–79. doi:10.1038/352077a0
Lieberman AP, Harmison G, Strand AD, Olson JM, Fischbeck KH (2002) Altered transcriptional regulation in cells expressing the expanded polyglutamine androgen receptor. Hum Mol Genet 11:1967–1976
Loro E, Rinaldi F, Malena A, Masiero E, Novelli G, Angelini C, Romeo V, Sandri M, Botta A, Vergani L (2010) Normal myogenesis and increased apoptosis in myotonic dystrophy type-1 muscle cells. Cell Death Differ 17:1315–1324. doi:10.1038/cdd.2010.33
Mariotti C, Castellotti B, Pareyson D, Testa D, Eoli M, Antozzi C, Silani V, Marconi R, Tezzon F, Siciliano G, Marchini C, Gellera C, Donato SD (2000) Phenotypic manifestations associated with CAG-repeat expansion in the androgen receptor gene in male patients and heterozygous females: a clinical and molecular study of 30 families. Neuromuscul Disord 10:391–397
Mo K, Razak Z, Rao P, Yu Z, Adachi H, Katsuno M, Sobue G, Lieberman AP, Westwood JT, Monks DA (2010) Microarray analysis of gene expression by skeletal muscle of three mouse models of Kennedy disease/spinal bulbar muscular atrophy. PLoS ONE 5:e12922. doi:10.1371/journal.pone.0012922
Monks DA, Johansen JA, Mo K, Rao P, Eagleson B, Yu Z, Lieberman AP, Breedlove SM, Jordan CL (2007) Overexpression of wild-type androgen receptor in muscle recapitulates polyglutamine disease. Proc Natl Acad Sci USA 104:18259–18264. doi:10.1073/pnas.0705501104
Montie HL, Cho MS, Holder L, Liu Y, Tsvetkov AS, Finkbeiner S, Merry DE (2009) Cytoplasmic retention of polyglutamine-expanded androgen receptor ameliorates disease via autophagy in a mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet 18:1937–1950. doi:10.1093/hmg/ddp115
Nedelsky NB, Pennuto M, Smith RB, Palazzolo I, Moore J, Nie Z, Neale G, Taylor JP (2010) Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron 67:936–952. doi:10.1016/j.neuron.2010.08.034
Orr HT, Zoghbi HY (2007) Trinucleotide repeat disorders. Annu Rev Neurosci 30:575–621. doi:10.1146/annurev.neuro.29.051605.113042
Palazzolo I, Burnett BG, Young JE, Brenne PL, La Spada AR, Fischbeck KH, Howell BW, Pennuto M (2007) Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum Mol Genet 16:1593–1603. doi:10.1093/hmg/ddm109
Palazzolo I, Stack C, Kong L, Musaro A, Adachi H, Katsuno M, Sobue G, Taylor JP, Summer CJ, Fischbeck KH, Pennuto M (2009) Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron 63:316–328. doi:10.1016/j.neuron.2009.07.019
Pallafacchina G, Blaauw B, Schiaffino S (2012) Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr Metab Cardiovasc Dis. doi:10.1016/j.numecd.2012.02.002
Parodi S, Pennuto M (2011) Neurotoxic effects of androgens in spinal and bulbar muscular atrophy. Front Neuroendocrinol 32:416–425. doi:10.1016/j.yfrne.2011.06.003
Poletti A (2004) The polyglutamine tract of androgen receptor: from functions to dysfunctions in motor neurons. Front Neuroendocrinol 25:1–26. doi:10.1016/j.yfrne.2004.03.001
Pradat PF, Barani A, Wanschitz J, Dubourg O, Lombes A, Bigot A, Mouly V, Bruneteau G, Salachas F, Lenglet T, Meininger V, Butler-Browne G (2011) Abnormalities of satellite cells function in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 12:264–271. doi:10.3109/17482968.2011.566618
Ross CA (2002) Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington’s disease and related disorders. Neuron 35:819–822
Sakuma K, Yamaguchi A (2010) The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotechnol 2010:721219. doi:10.1155/2010/721219
Sambataro F, Pennuto M (2012) Cell-autonomous and non-cell-autonomous toxicity in polyglutamine diseases. Prog Neurobiol 97:152–172. doi:10.1016/j.pneurobio.2011.10.003
Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23:160–170. doi:10.1152/physiol.00041.2007
Sandri M, Carraro U (1999) Apoptosis of skeletal muscles during development and disease. Int J Biochem Cell Biol 31:1373–1390
Schmidt BJ, Greenberg CR, Allingham-Hawkins DJ, Spriggs EL (2002) Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology 59:770–772
Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S (2004) Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 89:5245–5255. doi:10.1210/jc.2004-0084
Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S (2002) Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab 283:E154–E164. doi:10.1152/ajpendo.00502.2001
Sobue G, Hashizume Y, Mukai E, Hirayama M, Mitsuma T, Takahashi A (1989) X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain 112(Pt 1):209–232
Soraru G, D’Ascenzo C, Polo A, Palmieri A, Baggio L, Vergani L, Gellera C, Moretto G, Pegoraro E, Angelini C (2008) Spinal and bulbar muscular atrophy: skeletal muscle pathology in male patients and heterozygous females. J Neurol Sci 264:100–105. doi:10.1016/j.jns.2007.08.012
Sorenson EJ, Klein CJ (2007) Elevated creatine kinase and transaminases in asymptomatic SBMA. Amyotroph Lateral Scler 8:62–64. doi:10.1080/17482960600765040
Vergani L, Martinuzzi A, Carelli V, Cortelli P, Montagna P, Schievano G, Carrozzo R, Angelini C, Lugaresi E (1995) MtDNA mutations associated with Leber’s hereditary optic neuropathy: studies on cytoplasmic hybrid (cybrid) cells. Biochem Biophys Res Commun 210:880–888. doi:10.1006/bbrc.1995.1740
Wannenes F, Caprio M, Gatta L, Fabbri A, Bonini S, Moretti C (2008) Androgen receptor expression during C2C12 skeletal muscle cell line differentiation. Mol Cell Endocrinol 292:11–19. doi:10.1016/j.mce.2008.05.018
Yu Z, Wang AM, Robins DM, Lieberman AP (2009) Altered RNA splicing contributes to skeletal muscle pathology in Kennedy disease knock-in mice. Dis Model Mech 2:500–507. doi:10.1242/dmm.003301
Yu Z, Dadgar N, Albertelli M, Gruis K, Jordan C, Robins DM, Lieberman AP (2006) Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model. J Clin Invest 116:2663–2672. doi:10.1172/JCI28773
Acknowledgments
We thank Prof. Egle Perissinotto at University of Padova for statistical analysis, Dr. Emiliano Pena for the collaboration in tissue culture. We are grateful to Prof. Stefano Schiaffino and Dr. Stefano Ciciliot at Venetian Institute of Molecular Medicine for the comments and assistance in discussion, to Dr. Flaviano Favaro and Dr. Diego Faggian at Azienda Ospedale Padova for the kind and efficient collaboration for IL-4 detection. Work supported by Association Française contre les Myopathies (14073 and 14927 to GS, 14631 to LV), Telethon-Italy (GGP10145 to LV; GGP10037 to MP), Progetto d’Ateneo-Università di Padova (to GS). AM was supported by University of Padova, Italy.
Conflict of interest
MP received support from Siena Biotech (Italy).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2013_1122_MOESM1_ESM.tif
Supplementary Figure 1. Phosphorylated Akt/Akt ratio in DHT-treated myoblasts and T10 myotubes. (a) Representative WB analysis for phosphorylated Akt (pAkt) and Akt in total cell lysates from DHT-treated myoblasts and T10 myotubes. (b) Ratio of pAkt/total Akt in SBMA myoblasts and T10 myotubes is similar to control. The diagram represents the values expressed as mean ± SD of three independent experiments. The number of single lines studied is given in brackets (TIFF 1180 kb)
401_2013_1122_MOESM2_ESM.tif
Supplementary Figure 2. Apoptotic features in DHT-treated T10 myotubes. (a) Representative images of TUNEL assay. Scale bar, 15 μm. (b) Similar number of TUNEL positive nuclei per myotubes in control and SBMA myotubes; values were obtained in at least 50 myotubes/cell line. The number of single lines studied is given in brackets (TIFF 1724 kb)
Rights and permissions
About this article
Cite this article
Malena, A., Pennuto, M., Tezze, C. et al. Androgen-dependent impairment of myogenesis in spinal and bulbar muscular atrophy. Acta Neuropathol 126, 109–121 (2013). https://doi.org/10.1007/s00401-013-1122-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1122-9