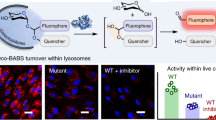
Abstract
Alpha-galactosidase A hydrolyzes the terminal alpha-galactosyl moieties from glycolipids and glycoproteins in lysosomes. Mutations in α-galactosidase cause lysosomal accumulation of the glycosphingolipid, globotriaosylceramide, which leads to Fabry disease. Small-molecule chaperones that bind to mutant enzyme proteins and correct their misfolding and mistrafficking have emerged as a potential therapy for Fabry disease. We have synthesized a red fluorogenic substrate, resorufinyl α-d-galactopyranoside, for a new α-galactosidase enzyme assay. This assay can be measured continuously at lower pH values, without the addition of a stop solution, due to the relatively low pK a of resorufin (~6). In addition, the assay emits red fluorescence, which can significantly reduce interferences due to compound fluorescence and dust/lint as compared to blue fluorescence. Therefore, this new red fluorogenic substrate and the resulting enzyme assay can be used in high-throughput screening to identify small-molecule chaperones for Fabry disease.






Similar content being viewed by others
References
Desnick R, Ioannou Y, Eng C (2001) Alpha-galactosidase A deficiency: Fabry disease. In: Scriver CR, Baudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, vol 1, 8th edn. McGraw-Hill, New York, pp 3733–3774
Ishii S et al (2007) Mutant alpha-galactosidase A enzymes identified in Fabry disease patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochem J 406(2):285–295
Ishii S, Suzuki Y, Fan JQ (2000) Role of Ser-65 in the activity of alpha-galactosidase A: characterization of a point mutation (S65T) detected in a patient with Fabry disease. Arch Biochem Biophys 377(2):228–233
Ishii S et al (1993) Characterization of a mutant alpha-galactosidase gene product for the late-onset cardiac form of Fabry disease. Biochem Biophys Res Commun 197(3):1585–1589
Brady RO (2006) Enzyme replacement for lysosomal diseases. Annu Rev Med 57:283–296
Lidove O et al (2007) Clinical results of enzyme replacement therapy in Fabry disease: a comprehensive review of literature. Int J Clin Pract 61(2):293–302
Wilcox WR et al (2004) Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet 75(1):65–74
Moore DF et al (2001) Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation 104(13):1506–1512
Bernier V, Bichet DG, Bouvier M (2004) Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol 4(5):528–533
Ulloa-Aguirre A et al (2004) Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 5(11):821–837
Fan JQ, Ishii S (2007) Active-site-specific chaperone therapy for Fabry disease. Yin and yang of enzyme inhibitors. Febs J 274(19):4962–4971
Shin SH et al (2007) Screening for pharmacological chaperones in Fabry disease. Biochem Biophys Res Commun 359(1):168–173
Hultberg B, Sjoblad S, Ockerman PA (1975) Glycosidases in human skin fibroblast cultures. Alpha-fucosidase, alpha-galactosidase, alpha-glucosidase, beta-mannosidase, and N-acetyl-alpha-glucosaminidase. Acta Paediatr Scand 64(1):123–131
Mapes CA, Sweeley CC (1973) Galactosyl (alpha 1–4) galactosylceramide: galactosyl hydrolase activity in normal and Fabry plasma. Biochem Biophys Res Commun 53(4):1317–1324
de Groot PG et al (1978) A new immunochemical method for the quantitative measurement of specific gene products in man-rodent somatic cell hybrids. Hum Genet 44(3):295–304
Yagi F, Eckhardt AE, Goldstein IJ (1990) Glycosidases of Ehrlich ascites tumor cells and ascitic fluid—purification and substrate specificity of alpha-N-acetylgalactosaminidase and alpha-galactosidase: comparison with coffee bean alpha-galactosidase. Arch Biochem Biophys 280(1):61–67
Tsou KC, Su HC (1964) A study of yeast alpha-galactosidase with naphthyl alpha-d-galactopyranosides as chromogenic substrates. Anal Biochem 8:415–423
Simeonov A et al (2008) Fluorescence spectroscopic profiling of compound libraries. J Med Chem 51(8):2363–2371
Acknowledgments
This research was supported by the Molecular Libraries Initiative of the NIH Roadmap for Medical Research and the Intramural Research Programs of the National Human Genome Research Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhen-Dan Shi and Omid Motabar contributed equally to this work
Rights and permissions
About this article
Cite this article
Shi, ZD., Motabar, O., Goldin, E. et al. Synthesis and characterization of a new fluorogenic substrate for alpha-galactosidase. Anal Bioanal Chem 394, 1903–1909 (2009). https://doi.org/10.1007/s00216-009-2879-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-2879-5