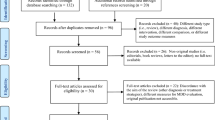
Abstract
Rationale
Dysregulation of the one carbon cycle is documented in depression. Thereby, S-adenosylmethionine (SAMe), a one–carbon cycle nutraceutical compound with a favourable side effect profile, has a theoretical rationale for efficacy. However, further controlled studies are required to confirm SAMe’s efficacy.
Objectives
To test the efficacy of SAMe versus placebo in unmedicated DSM-5 diagnosed (major depressive disorder) (MDD) patients with mild-to-moderate levels of depressive symptoms.
Methods
We conducted an 8-week, double-blind, randomised controlled trial testing 800 mg/day of SAMe monotherapy versus placebo in 49 patients with MDD (Montgomery-Åsberg Depression Rating Scale [MADRS] score 14–25) who were not currently taking antidepressants. One–carbon cycle biomarkers, brain-derived neurotropic factor (BDNF), and relevant single nucleotide polymorphisms (SNPs) were analysed as potential treatment moderators.
Results
A clinically relevant differential reduction from baseline to week 8 of 3.76 points occurred on the primary outcome (MADRS) in favour of SAMe. This however was not significant (p = 0.13) on an adjusted linear mixed model, notwithstanding a medium to large effect size of 0.72. A high placebo response rate of 53% occurred (> 50% reduction on MADRS). Exploratory analyses showed that SAMe was however effective in reducing depression amongst participants with milder depression severity (MADRS ≤ 22, p = 0.045). Response was not moderated by BDNF, SNPs, or one–carbon cycle biomarkers, although increased folate concentrations were correlated with improved symptoms in the SAMe group (r = − 0.57, p = 0.026). The treatment was safe and well tolerated.
Conclusions
Although a differential reduction in depression symptoms between groups was observed in favour of SAMe, the results of this pilot study were not statistically significant.
Trial registration
ANZCTR—Australian New Zealand Clinical Trials Registry; No.: ACTRN12613001299796; URL: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364900


Similar content being viewed by others
References
Arnold O, Saletu B, Anderer P et al (2005) Double-blind, placebo-controlled pharmacodynamic studies with a nutraceutical and a pharmaceutical dose of ademetionine (SAMe) in elderly subjects, utilizing EEG mapping and psychometry. Eur Neuropsychopharmacol 15:533–543. https://doi.org/10.1016/j.euroneuro.2005.01.004
Beck AT, Ward C, Mendelson M (1961) Beck depression inventory (BDI). Arch Gen Psychiatry 4:561–571
Cuthbert BN, Insel TR (2013) Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 11. https://doi.org/10.1186/1741-7015-11-126
De Sousa DP, Silva RHN, Da Silva EF, Gavioli EC (2017) Essential oils and their constituents: an alternative source for novel antidepressants. Molecules. 22. https://doi.org/10.3390/molecules22081290
Desseilles M, Witte J, Chang TE, Iovieno N, Dording C, Ashih H, Nyer M, Freeman MP, Fava M, Mischoulon D (2013) Massachusetts general hospital SAFER criteria for clinical trials and research. Harv Rev Psychiatry 21:269–274. https://doi.org/10.1097/HRP.0b013e3182a75cc7
Dunkley EJ, Isbister GK, Sibbritt D et al (2003) The hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 96:635–642
Duru G, Fantino B (2008) The clinical relevance of changes in the Montgomery–Asberg depression rating scale using the minimum clinically important difference approach. Curr Med Res Opin 24:1329–1335. https://doi.org/10.1185/030079908X291958
Frodl T (2017) Recent advances in predicting responses to antidepressant treatment. F1000Research. https://doi.org/10.12688/f1000research.10300.1
Galizia I, Oldani L, Macritchie K, Amari E, Dougall D, Jones TN, Lam RW, Massei GJ, Yatham LN, Young AH, Cochrane Common Mental Disorders Group (2016) S-adenosyl methionine (SAMe) for depression in adults. Cochrane Database Syst Rev
Guy W (1976) Clinical global impression scale. ECDEU assess man psychopharmacol Vol DHEW Publ No ADM 76 338:218–222
Hamilton M (1959) Hamilton anxiety rating scale (HAM-A). J Med 61:81–82. https://doi.org/10.1145/363332.363339
Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, Iversen M, Banke MB, Petersen IJ, Klingenberg SL, Krogh J, Ebert SE, Timm A, Lindschou J, Gluud C (2017) Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry 17. https://doi.org/10.1186/s12888-016-1173-2
Khan A, Leventhal RM, Khan SR, Brown WA (2002) Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol 22:40–45
Levine J, Schooler NR (1986) SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull 22:343–381. https://doi.org/10.1093/jnci/djm189
Mischoulon D, Price LH, Carpenter LL, Tyrka AR, Papakostas GI, Baer L, Dording CM, Clain AJ, Durham K, Walker R, Ludington E, Fava M (2014) A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder. J Clin Psychiatry 75:370–376. https://doi.org/10.4088/JCP.13m08591
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Parrott AC, Hindmarch I (1980) The leeds sleep evaluation questionnaire in psychopharmacological investigations-a review. Psychopharmacology 71:173–179. https://doi.org/10.1007/BF00434408
Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A (2011) Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol 21:841–860. https://doi.org/10.1016/j.euroneuro.2011.04.002
Sarris J, Papakostas GI, Vitolo O et al (2014) S-Adenosyl methionine (SAMe) versus escitalopram and placebo in major depression: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord 164:76–81
Sarris J, Price LH, Carpenter LL, Tyrka A, Ng C, Papakostas G, Jaeger A, Fava M, Mischoulon D (2015) Is S-adenosyl methionine (SAMe) for depression only effective in males? A re-analysis of data from a randomized clinical trial. Pharmacopsychiatry 48:141–144. https://doi.org/10.1055/s-0035-1549928
Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH (2016) Adjunctive nutrient nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 173:575–587
Sarris J, Byrne GJ, Bousman C, Stough C, Murphy J, MacDonald P, Adams L, Nazareth S, Oliver G, Cribb L, Savage K, Menon R, Chamoli S, Berk M, Ng C, Mischoulon D (2018) Adjunctive S-adenosylmethionine (SAMe) in treating non-remittent major depressive disorder: an 8-week double-blind, randomized, controlled trial. Eur Neuropsychopharmacol 28:1126–1136. https://doi.org/10.1016/j.euroneuro.2018.07.098
Sharma A, Gerbarg P, Bottiglieri T, Massoumi L, Carpenter LL, Lavretsky H, Muskin PR, Brown RP, Mischoulon D, as Work Group of the American Psychiatric Association Council on Research (2017) S-adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry 78:e656–e667
Sheehan DV, Lecrubier Y, Sheehan KH et al (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:34–57
Sternbach H (1991) The serotonin syndrome. Am J Psychiatry 148:705–713. https://doi.org/10.1176/ajp.148.6.705
Ware JE, Kosinski M, Keller SD (1996) A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233. https://doi.org/10.1097/00005650-199603000-00003
Acknowledgments
We would like to thank the study participants who contributed to this project.
Funding
This study is funded by a National Health and Medical Research Council (NHMRC) project grant (APP1048222), and is co-sponsored by FIT-BioCeuticals. Jerome Sarris is supported by an NHMRC Fellowship (APP1125000). Michael Berk is supported by a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (APP1059660 and APP1156072). Study sponsors had no role in study design, in the collection and analysis of data, manuscript preparation, nor the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
Author Sarris designed the study. Authors Sarris, Byrne, Bousman, Stough, Berk, Ng, Chamoli, Menon, and Mischoulon contributed to the design and write-up of the protocol. Authors Sarris, Bousman, and Cribb designed and implemented the statistical analysis. Authors Murphy, Cribb, MacDonald, Adams, Nazareth, Oliver, and Savage were involved in the data collection and entry. All authors have contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
The trial had ethics approval (TMC REC: 232; UQ MREC: 2014000702) and is registered with ANZCTR (protocol number: 12613001299796).
Conflict of interest
JS has received honoraria, research support, royalties, or consultancy or travel grant funding from Integria Healthcare & MediHerb, Pfizer, Scius Health, Key Pharmaceuticals, Taki Mai, FIT-BioCeuticals, Blackmores, Soho-Flordis, Australian Natural Therapeutics Group, Grünbiotics, Healthworld, HealthEd, HealthMasters, SPRIM, Elsevier, Chaminade University, International Society for Affective Disorders, Complementary Medicines Australia, Terry White Chemists, ANS, Society for Medicinal Plant and Natural Product Research, Sanofi-Aventis, Omega-3 Centre, the National Health and Medical Research Council, and CR Roper Fellowship. DM has received research support from Nordic Naturals. He has received honoraria for consulting, speaking, and writing from the Massachusetts General Hospital Psychiatry Academy, Blackmores, and PeerPoint Medical Education Institute, LLC. He has provided unpaid consulting for Pharmavite LLC and Gnosis USA, Inc. He has received royalties from Lippincott Williams & Wilkins for published book ‘Natural Medications for Psychiatric Disorders: Considering the Alternatives.’ MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Rotary Health, Geelong Medical Research Foundation, Bristol-Myers Squibb, Eli Lilly, Glaxo SmithKline, Meat and Livestock Board, Organon, Novartis, Mayne Pharma, Servier, Woolworths, Avant, and the Harry Windsor Foundation; has been a speaker for Astra Zeneca, Bristol-Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen-Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay, and Wyeth; and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Eli Lilly, Grunbiotics, Glaxo SmithKline, Janssen-Cilag, LivaNova, Lundbeck, Merck, Mylan, Otsuka, Pfizer, and Servier. CS has received research grants from Government: Australian Research Council; NHMRC; NDLERF; NIDA; VDLERF; ARC; Vicpolice; Vicroads; Nestle; SFI; Barry Callebaut; Blackmores; Flordis; Zeller; Clover Corporation; Horphag; GSK; Bayer; Pharmalink; Siemens; Securatek; Efamol; Cognis; Integria; Medvet, Cooper Nutrition, and paid strategic consulting advice for SFI, Blackmores, Bayer; ARU, Carlton FC, GSK India. DM has received research support from Nordic Naturals. He has provided unpaid consulting for Pharmavite LLC and Gnosis USA, Inc. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy, from PeerPoint Medical Education Institute LLC, and from Blackmores. He has received royalties from Lippincott Williams & Wilkins for published book ‘Natural Medications for Psychiatric Disorders: Considering the Alternatives.’ GJB receives research support from Janssen-Cilag, Eli Lilly, Biogen, NHMRC (APP1104460, APP1095227, APP1063383, APP1048222) and the RBWH Foundation. He owns shares in CSL. He receives royalties from the sale of his books Community Mental Health for Older People and Psychosocial Dimensions of Medicine. He receives licensing fees from the use of his rating scale, the Geriatric Anxiety Inventory. He is employed by the University of Queensland, the Royal Brisbane & Women’s Hospital, and the Repatriation Medical Authority. CN had served as a consultant for Lundbeck, Grunbiotics, Servier, Janssen-Cilag, Wyeth and Eli Lilly, received research grant support from Wyeth and Lundbeck, and speaker honoraria from Servier, Lundbeck, Bristol-Myers Squibb, Organon, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Astra-Zenaca, Wyeth, and Pfizer.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarris, J., Murphy, J., Stough, C. et al. S-Adenosylmethionine (SAMe) monotherapy for depression: an 8-week double-blind, randomised, controlled trial. Psychopharmacology 237, 209–218 (2020). https://doi.org/10.1007/s00213-019-05358-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05358-1