Abstract
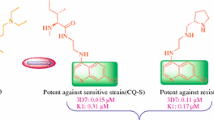
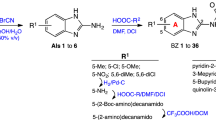
A series of novel imidazoisoquinolinone derivatives were synthesized and evaluated for in vitro antimalarial efficacy against chloroquine sensitive GHA strain of Plasmodium falciparum. Compounds 2, 4, 6, 9, and 17 revealed moderate to good activities in the micromolar range. Binding interaction between these active compounds and heme were determined and correlated with antimalarial activity. A good correlation (r = 0.98) was observed between antimalarial activity and the heme dissociation constants (K d). These suggest that antimalarial mode of action of this class of compounds appears to be similar to that of chloroquine and involves the inhibition of hemozoin formation.






Similar content being viewed by others
References
Acharya BN, Saraswat D, Tiwari M, Shrivastava AK, Ghorpade R, Bapna S, Kaushik MP (2010) Synthesis and antimalarial evaluation of 1,3,5-trisubstituted pyrazolines. Eur J Med Chem 45(2):430–438. doi:10.1016/j.ejmech.2009.10.023
Allen CFHB (1955) Organic Synthesis, 3
Bollini M, Asís SE, Bruno AM (2006) Synthesis of 2,3-dihydroimidazo[1,2-b]isoquinoline-5(1H)-one and derivatives. Synthesis 2006:237–242. doi:10.1055/s-2005-918505
Bollini M, Casal JJ, Alvarez DE, Boiani L, Gonzalez M, Cerecetto H, Bruno AM (2009) New potent imidazoisoquinolinone derivatives as anti-Trypanosoma cruzi agents: biological evaluation and structure-activity relationships. Bioorg Med Chem 17(4):1437–1444. doi:10.1016/j.bmc.2009.01.011
Carrër A, Florent J-C, Auvrouin E, Rousselle P, Bertounesque E (2011) Synthesis of 3-aryl-2-arylamidobenzofurans based on the curtius rearrangement. J Org Chem 76(8):2502–2520. doi:10.1021/jo102265b
Chevli R, Fitch CD (1982) The antimalarial drug mefloquine binds to membrane phospholipids. Antimicrob Agents Chemother 21(4):581–586
Curtius T (1890) Hydrazoic acid. Ber 23:3023–3041
Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE (2000) Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell 6(4):861–871
Fitch CD (2004) Ferriprotoporphyrin IX, phospholipids, and the antimalarial actions of quinoline drugs. Life Sci 74(16):1957–1972. doi:10.1016/j.lfs.2003.10.003
Gorka AP, Alumasa JN, Sherlach KS, Jacobs LM, Nickley KB, Brower JP, de Dios AC, Roepe PD (2013) Cytostatic versus cytocidal activities of chloroquine analogues and inhibition of hemozoin crystal growth. Antimicrob Agents Chemother 57(1):356–364. doi:10.1128/AAC.01709-12
Guantai EM, Ncokazi K, Egan TJ, Gut J, Rosenthal PJ, Smith PJ, Chibale K (2010) Design, synthesis and in vitro antimalarial evaluation of triazole-linked chalcone and dienone hybrid compounds. Bioorg Med Chem 18(23):8243–8256. doi:10.1016/j.bmc.2010.10.009
Joshi AA, Viswanathan CL (2006) Docking studies and development of novel 5-heteroarylamino-2,4-diamino-8-chloropyrimido-[4,5-b]quinolines as potential antimalarials. Bioorg Med Chem Lett 16(10):2613–2617. doi:10.1016/j.bmcl.2006.02.038
Kumar S, Das SK, Dey S, Maity P, Guha M, Choubey V, Panda G, Bandyopadhyay U (2008) Antiplasmodial activity of [(aryl)arylsulfanylmethyl]pyridine. Antimicrob Agents Chemother 52(2):705–715. doi:10.1128/AAC.00898-07
Muscia GC, Cazorla SI, Frank FM, Borosky GL, Buldain GY, Asís SE, Malchiodi EL (2011) Synthesis, trypanocidal activity and molecular modeling studies of 2-alkylaminomethylquinoline derivatives. Eur J Med Chem 46(9):3696–3703. doi:10.1016/j.ejmech.2011.05.035
Nagarajan K, Rao VR, Shah RK, Shenoy SJ, Fritz H, Richter WJ, Muller D (1988) Condensed heterotricycles. Synthesis and reactions of b-fused 1(2H)-isoquinolinones with unusual enaminic properties. Helv Chim Acta 71(1):77–92. doi:10.1002/hlca.19880710110
O’Neill PM, Willock DJ, Hawley SR, Bray PG, Storr RC, Ward SA, Park BK (1997) Synthesis, antimalarial activity, and molecular modeling of tebuquine analogues. J Med Chem 40(4):437–448. doi:10.1021/jm960370r
Porcal W, Hernández P, Aguirre G, Boiani L, Boiani M, Merlino A, Ferreira A, Maio RD, Castro A, González M, Cerecetto H (2007) Second generation of 5-ethenylbenzofuroxan derivatives as inhibitors of Trypanosoma cruzi growth: synthesis, biological evaluation, and structure–activity relationships. Bioorg Med Chem 15(7):2768–2781. doi:10.1016/j.bmc.2007.01.009
Taylor DK, Avery TD, Greatrex BW, Tiekink ER, Macreadie IG, Macreadie PI, Humphries AD, Kalkanidis M, Fox EN, Klonis N, Tilley L (2004) Novel endoperoxide antimalarials: synthesis, heme binding, and antimalarial activity. J Med Chem 47(7):1833–1839. doi:10.1021/jm0305319
Wavefunction IVKA, Suite 370, Irvine, Calfornia 92612, USA
WHO. http://www.who.int/tdr/, for in vitro protocols and activity criteria
Wiberg KB (1986) Ab Initio Molecular Orbital Theory by W. J. Hehre, L. Radom, P. V. R. Schleyer, and J. A. Pople, John Wiley, New York, 548 pp. (1986). J Comput Chem 7(3):379. doi:10.1002/jcc.540070314
Xu Kelly J, Winter R, Riscoe M, Peyton DH (2001) A spectroscopic investigation of the binding interactions between 4,5-dihydroxyxanthone and heme. J Inorg Biochem 86(2–3):617–625. doi:10.1016/S0162-0134(01)00217-3
Acknowledgments
We thank Tropical Disease Research (TDR) Program,World Health Organization (WHO, Switzerland) for in vitro malaria assays. This work was supported by a Grant from University of Buenos Aires (UBACyT B001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bollini, M., Casal, J.J., Asís, S.E. et al. Antimalarial activity of novel imidazoisoquinolinone derivatives correlates with heme binding affinity. Med Chem Res 24, 1496–1503 (2015). https://doi.org/10.1007/s00044-014-1231-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1231-6