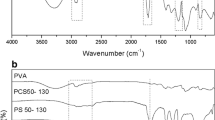
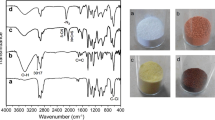
Abstract
Four porous polymeric supports prepared from epoxy monomers have been analyzed with respect to their catalytic behavior and characterized by means of infrared spectroscopy (FTIR-ATR), nitrogen adsorption and Inverse Gas Chromatography at Infinite Dilution (IGC-ID). The Pseudomonas stutzeri lipase has been used to determine the adsorption and desorption rates on each support. It has been found that the specific surface area increases with the number of epoxy groups and the pore volume increases as well. The surface energies are also related with the number of epoxy groups in the monomer and show a decrease in the dispersive component of the surface energy, γ dS , from 104.87 to 71.12 mJ m−2, but an increase in the polar or acid–base characteristics. Regarding the adsorption–desorption rates of the enzyme lipase, it occurs in two stages being the short-time processes controlled by the dispersive surface energy of the polymer, but the desorption rate is opposite to the base-to-acid surface energy ratio of the polymer indicating that for long-time processes the enzyme is attached to the polymer surface mainly by acid–base interactions.









Similar content being viewed by others
References
Hanefeld U, Gardossi L, Magner E (2009) Understanding enzyme immobilisation. Chem Soc Rev 38(2):453–468. doi:10.1039/b711564b
Villeneuve P, Muderhwa JM, Graille J, Haas MJ (2000) Customizing lipases for biocatalysis: a survey of chemical, physical and molecular biological approaches. J Mol Catal B Enzym 9(4–6):113–148. doi:10.1016/s1381-1177(99)00107-1
Káňavová N, Kosior A, Antošová M, Faber R, Polakovič M (2012) Application of a micromembrane chromatography module to the examination of protein adsorption equilibrium. J Sep Sci 35(22):3177–3183. doi:10.1002/jssc.201200396
Bickerstaff GF (1996) Methods in biotechnology—immobilization of enzymes and cells. Humana Press, New Jersey
Chibata I, Tosa T, Sato T (1986) Biocatalysis—immobilized cells and enzymes. J Mol Catal 37(1):1–24. doi:10.1016/0304-5102(86)85134-3
Hartmeier W (1985) Immobilized biocatalyst—From simple to complex systems. Trends Biotechnol 3(6):149–153. doi:10.1016/0167-7799(85)90104-0
Katchalski-Katzir E (1993) Immobilized enzymes—learning from past successes and failures. Trends Biotechnol 11(11):471–478. doi:10.1016/0167-7799(93)90080-s
Cheetham PSJ (1993) The use of biotransformations for the production of flavors and fragrances. Trends Biotechnol 11(11):478–488. doi:10.1016/0167-7799(93)90081-j
de Oliveira PC, Alves GM, de Castro HF (2000) Immobilisation studies and catalytic properties of microbial lipase onto styrene-divinylbenzene copolymer. Biochem Eng J 5(1):63–71. doi:10.1016/S1369-703X(99)00061-3
Basri M, Yunus W, Yoong WS, Ampon K, Razak CNA, Salleh AB (1996) Immobilization of lipase from Candida rugosa on synthetic polymer beads for use in the synthesis of fatty esters. J Chem Technol Biotechnol 66(2):169–173. doi:10.1002/(sici)1097-4660(199606)66:2
Arica MY, Halicigil C, Alaeddinoglu G, Denizli A (1999) Affinity interaction of hydroxypyruvate reductase from Methylophilus spp. with Cibacron blue F3GA-derived poly(HEMA EGDMA) microspheres: partial purification and characterization. Process Biochem 34(4):375–381. doi:10.1016/S0032-9592(98)00104-6
Arica MY, Testereci HN, Denizli A (1998) Dye-ligand and metal chelate poly(2-hydroxyethylmethacrylate) membranes for affinity separation of proteins. J Chromatogr A 799(1–2):83–91. doi:10.1016/s0021-9673(97)01079-0
Balcao VM, Paiva AL, Malcata FX (1996) Bioreactors with immobilized lipases: State of the art. Enzym Microb Technol 18(6):392–416. doi:10.1016/0141-0229(95)00125-5
Arica MY (2000) Epoxy-derived pHEMA membrane for use bioactive macromolecules immobilization: Covalently bound urease in a continuous model system. J Appl Polym Sci 77(9):2000–2008. doi:10.1002/1097-4628(20000829)77:9
Lozinsky VI (2008) Polymeric cryogels as a new family of macroporous and supermacroporous materials for biotechnological purposes. Russ Chem Bull 57(5):1015–1032. doi:10.1007/s11172-008-0131-7
Arshady R (1991) Beaded polymer suports and gels. 1. Manufacturing techniques. J Chromatogr 586(2):181–197. doi:10.1016/0021-9673(91)85124-x
Wheatley JB, Schmidt DE (1999) Salt-induced immobilization of affinity ligands onto epoxide-activated supports. J Chromatogr A 849(1):1–12. doi:10.1016/s0021-9673(99)00484-7
Katchalski-Katzir E, Kraemer DM (2000) Eupergit (R) C, a carrier for immobilization of enzymes of industrial potential. J Mol Catal B Enzym 10(1–3):157–176. doi:10.1016/s1381-1177(00)00124-7
Mateo C, Torres R, Fernandez-Lorente G, Ortiz C, Fuentes M, Hidalgo A, Lopez-Gallego F, Abian O, Palomo JM, Betancor L, Pessela BCC, Guisan JM, Fernandez-Lafuente R (2003) Epoxy-amino groups: a new tool for improved immobilization of proteins by the epoxy method. Biomacromolecules 4(3):772–777. doi:10.1021/bm0257661
Hannibal-Friedrich O, Chun M, Sernetz M (1980) Immobilization of beta-galactosidase, albumin and gamma-globulin on epoxy-activated acrylic beads. Biotechnol Bioeng 22(1):157–175. doi:10.1002/bit.260220112
Smalla K, Turkova J, Coupek J, Hermann P (1988) Influence of salts on the covalent immobilization of proteins to modified copolymers of 2-hydroxymethyl methacrylate with ethylene dimethacrylate. Biotechnol Appl Biochem 10(1):21–31. doi:10.1111/j.1470-8744.1988.tb00003.x
Melander WR, Corradini D, Horvath C (1984) Salt-mediated retention of proteins in hydrophobic-interaction chromatography—applications of solvophobic theory. J Chromatogr 317:67–85. doi:10.1016/s0021-9673(01)91648-6
Mateo C, Fernandez-Lorente G, Abian O, Fernandez-Lafuente R, Guisan JM (2000) Multifunctional epoxy supports: a new tool to improve the covalent immobilization of proteins. The promotion of physical adsorptions of proteins on the supports before their covalent linkage. Biomacromolecules 1(4):739–745. doi:10.1021/bm000071q
Kotha A, Raman RC, Ponrathnam S, Kumar KK, Shewale JG (1996) Beaded reactive polymers 2. Immobilisation of penicillin G acylase on glycidyl methacrylate divinyl benzene copolymers of differing pore size and its distribution. React Funct Polym 28(3):235–242. doi:10.1016/1381-5148(95)00089-5
Kotha A, Raman RC, Ponrathnam S, Shewale JG (1996) Beaded reactive polymers 1. Effect of synthesis variables on pore size and its distribution in beaded glycidyl methacrylate-divinyl benzene copolymers. React Funct Polym 28(3):227–233. doi:10.1016/1381-5148(95)00081-X
Vaidya BK, Karale AJ, Suthar HK, Ingavle G, Pathak TS, Ponrathnam S, Nene S (2007) Immobilization of mushroom polyphenol oxidase on poly(allyl glycidyl ether-co-ethylene glycol dimethacrylate) macroporous beaded copolymers. React Funct Polym 67(10):905–915. doi:10.1016/j.reactfunetpolym.2007.05.015
Yasuda M, Kobayashi M, Kotani T, Kawahara K, Nikaido H, Ueda A, Ogino H, Ishikawa H (2002) Synthesis of amphiphilic polymer particles by seed polymerization and their application for lipase immobilization. Macromol Chem Phys 203(2):284–293. doi:10.1002/1521-3935(20020101)203:2
Vaidya BK, Ingavle GC, Ponrathnam S, Kulkarni BD, Nene SN (2008) Immobilization of Candida rugosa lipase on poly(allyl glycidyl ether-co-thylene glycol dimethacrylate) macroporous polymer particles. Bioresour Technol 99(9):3623–3629. doi:10.1016/j.biortech.2007.07-035
Aires-Trapote A (2012) Diseño y síntesis de soportes poliméricos porosos para la obtención de nuevos biocatalizadores y su aplicación en procesos de química sostenible. Universidad Autonoma de Madrid, Madrid
Ansari DM, Price GJ (2004) Correlation of mechanical properties of clay filled polyamide mouldings with chromatographically measured surface energies. Polymer 45(11):3663–3670. doi:10.1016/j.polymer.2004.03.045
Čerović LS, Milonjić SK, Hourlier-Bahloul D (2005) Adsorption of n-alkanes on silicon nitride nanopowder. J Am Ceram Soc 88(7):1875–1878. doi:10.1111/j.1551-2916.2005.00371.x
Peña-Alonso R, Tamayo A, Rubio F, Rubio J (2005) Influence of boron concentration on the surface properties of TEOS-PDMS hybrid materials. J Sol Gel Sci Technol 36(1):113–124. doi:10.1007/s10971-005-2498-3
Martos C, Rubio F, Rubio J, Oteo JL (2001) Surface Energy of Silica-TEOS-PDMS Ormosils. J Sol Gel Sci Technol 20(2):197–210. doi:10.1023/a:1008759708396
Pena-Alonso R, Tellez L, Rubio J, Rubio F (2006) Surface chemical and physical properties of TEOS-TBOT-PDMS hybrid materials. J Sol Gel Sci Technol 38(2):133–145. doi:10.1007/s10971-006-7116-6
Tamayo A, Tellez L, Pena-Alonso R, Rubio F, Rubio J (2009) Surface changes during pyrolytic conversion of hybrid materials to oxycarbide glasses. J Mater Sci 44(21):5743–5753. doi:10.1007/s10853-009-3805-0
Tamayo A, Tellez L, Rubio J, Rubio F, Oteo JL (2010) Effect of reaction conditions on surface properties of TEOS-TBOT-PDMS hybrid materials. J Sol Gel Sci Technol 55(1):94–104. doi:10.1007/s10971-010-2220-y
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72(1–2):248–254. doi:10.1006/abio.1976.9999
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity. Academic Press, London
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73(1):373–380. doi:10.1021/ja01145a126
Avnir D, Farin D, Pfeifer P (1985) Surface geometric irregularity of particulate materials: the fractal approach. J Colloid Interface Sci 103(1):112–123. doi:10.1016/0021-9797(85)90082-7
Rubio F, Rubio J, Oteo JL (1997) Effect of heating on the surface fractal dimensions of ZrO2. J Mater Sci Lett 16(1):49–52. doi:10.1023/a:1018592532337
Ismail IMK, Pfeifer P (1994) Fractal analysis and surface roughness of non porous carbon fibers and carbon blacks. Langmuir 10(5):1532–1538. doi:10.1021/la00017a035
Neimark AV, Unger KK (1993) Method of discrimination of surface fractality. J Colloid Interface Sci 158(2):412–419. doi:10.1006/jcis.1993.1273
Jaroniec M (1995) Evaluation of the fractal dimension from a single adsorption isotherm. Langmuir 11(6):2316–2317. doi:10.1021/la00006a076
Gutmann V (1978) The donor–acceptor approach to molecular interactions. Plenum Press, New York
Riddle FL, Fowkes FM (1990) Spectral shifts in acid-base chemistry. 1. van der Waals contributions to acceptor numbers. J Am Chem Soc 112(9):3259–3264. doi:10.1021/ja00165a001
van Asten A, van Veenendaal N, Koster S (2000) Surface characterization of industrial fibers with inverse gas chromatography. J Chromatogr A 888(1–2):175–196. doi:10.1016/s0021-9673(00)00487-8
Conley RT (1972) Infrared spectroscopy. Allyn and Bacon, Boston
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619. doi:10.1351/pac198557040603
Harkins WD, Jura G (1944) Surfaces of solids XIII A vapor adsorption method for the determination of the area of a solid without the assumption of a molecular area, and the areas occupied by nitrogen and other molecules on the surface of a solid. J Am Chem Soc 66:1366–1373. doi:10.1021/ja01236a048
Lee CK, Tsay CS (1998) Surface fractal dimensions of alumina and aluminum borate from nitrogen isotherms. J Phys Chem B 102(21):4123–4130. doi:10.1021/jp9803485
El Shafei GMS, Philip CA, Moussa NA (2004) Fractal analysis of hydroxyapatite from nitrogen isotherms. J Colloid Interface Sci 277(2):410–416. doi:10.1016/j.jcis.2004.05.002
Fu J, He Q, Liu B, Zhang J, Hu B (2010) Study in adsorption behavior of polymer on molecular sieves by surface and pore properties. Colloids Surf A 369(1–3):113–120. doi:10.1016/j.colsurfa.2010.08.011
Sun CH, Berg JC (2003) A review of the different techniques for solid surface acid-base characterization. Adv Colloid Interface Sci 105:151–175. doi:10.1016/s0001-8686(03)00066-6
Thudi L, Jasti LS, Swarnalatha Y, Fadnavis NW, Mulani K, Deokar S, Ponrathnam S (2012) Enzyme immobilization on epoxy supports in reverse micellar media: Prevention of enzyme denaturation. J Mol Catal B Enzym 74(1–2):54–62. doi:10.1016/j.molcatb.2011.08.014
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamayo, A., Aires-Trapote, A., Rubio, F. et al. Effect of the surface parameters on the interaction of epoxy polymer supports with a lipase enzyme. Polym. Bull. 72, 195–218 (2015). https://doi.org/10.1007/s00289-014-1267-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1267-2